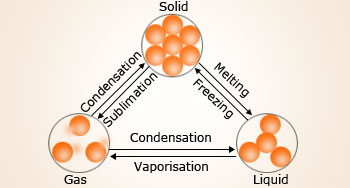
 Change of states of a substance or a compound
Melting: Solid matter changes its state to liquid.
Change of states of a substance or a compound
Melting: Solid matter changes its state to liquid.
Freezing: Liquid matter loses heat and changes its state to solid.
Boiling (vaporization): Liquid matter gains heat and changes its state to gas.
Condensation: Gas molecules lose heat and change its phase to liquid.
Sublimation: Solid matter changes directly to gas.
Ice melts and vaporizes quickly on hot griddle
Three states of H2O are Ice, water and vapor. 720 cal/gm is needed to first melt the solid, then vaporize the liquid water. The energy is absorbed by individual molecules of water to break the hydrogen bonds holding the crystal together resulting in a liquid and then a gas.
As you know, a molecule of water is made up of two hydrogen atoms and one oxygen atom. At ordinary temperatures, water is in liquid form. Take little amount of water in a container. If we heat the container to 100°C, the water inside will boil and become steam. The binding between each molecule of water is broken due to heat. Therefore the distance between each molecule becomes large. Steam is the gaseous state of water. If we reduce temperature, the steam will condense to become water again. Now, if we reduce the temperature of the container to 0°C, the water will solidify into ice. Thus the three states of matter for a water molecule are : ice in solid state, water in liquid state and steam in gaseous state.
It is good to remember that the states of matter depend on its temperature, the pressure that is exerted on it and transfer of energy or heat accompanying the change of state. Matter changes form when heat is supplied or removed.
The change from
solid  liquid
liquid  gas
gas
is called a change of state of a substance or a compound.
We know that solids are bound together by tight bonds. As we supply energy, the bonds start to stretch. This transforms the solid into liquid state. As more energy is given to the system, the bonds stretch even more and ultimately break. The liquid then is turned into gaseous matter. The inter–molecular forces become weaker as we go from solid to liquid to gaseous state.
The process of converting a solid into liquid is known as melting. The temperature at which melting occurs is called the melting point. The reverse of melting is called freezing or solidification. If solidification makes the solid into properly structured crystals, it is also known as crystallization process. The process of converting liquid into gas is called vaporizing. The reverse of vaporizing is known as condensation. The temperature at which the liquid turns into gas is called the boiling point of the substance.
Some solids like dry ice, iodine, frozen carbon dioxide, naphthalene balls convert to gaseous state directly from their solid state. They jump the liquid state. The process of going from solid state to gaseous state directly is known as sublimation. Reverse of sublimation is called condensation.