Qualitative analysis:
An acid and a base react to form a salt.
The salt consists of two parts known as radicals.
The cation or the positively charged part, derived from the base, called the basicradical
The anion or the negatively charged part derived from the acid termed as the acidicradical.
NaCl is a salt with basic radical Na+ and acidic radical Cl –.
In qualitative inorganic analysis ,the given compound is analysed for both the radicalsof the given salt
Characteristic tests of some acidic radicals or anions:
Preliminary tests:
1. Action of dil HCl and heat on the given salt :
- Brisk effervescence indicates presence of carbonate (CO3 2–) or sulphite(SO3 2– )
- Smell of rotten eggs indicates the presence of sulphide (S2–)
- Reddish brown gas (NO2 )indicates the presence of nitrite ( NO2– )
The above tests involve the following reactions:
| Acidic radical | Formula | Reaction |
|---|---|---|
| Carbonate | (CO3)2- | Na2CO3 + 2HCl → 2NaCl + H2O + CO2↑ |
| Sulphite | (SO3)2- | Na2SO3 + 2HCl → 2NaCl + H2O + SO2↑ |
| Sulphide | S2- | FeS + 2HCl → FeCl2 + H2S ↑ |
| Nitrite | NO2- | NaNO2 + HCl → NaCl +
HNO2 3HNO2 → HNO3 + 2NO↑ + H2O 1. 2NO + O2 → 2NO2↑ |
2. Action of conc H2SO4 and heat in a dry test tube :
- Colourless pungent gas (HCl) indicates chloride Cl –
- Brown gas (Br2) in cold condition , indicates bromide (Br – )
- Violet fumes (I2) indicates Iodide ( I – )
- Brown gas (NO2) on heating indicates NO3–
| Chloride | Cl- | 2NaCl + H2SO4 → Na2SO4 + 2HCl↑ |
| Bromide | Br- | 2KBr + 3H2SO4 → 2KHSO4 + 2H2O + SO2↑ + Br2↑ |
| Iodide | I- | 2KI + 3H2SO4 → 2KHSO4 + 2H2O + SO2 + I2↑ |
| Nitrate | NO3- | 4KNO3 + 4H2SO4 → 4KHSO4 + 2H2O + 4NO2↑ + O2↑ |
1.Tests for carbonate:
The carbonates of alkali metals and ammonium alone are water soluble.
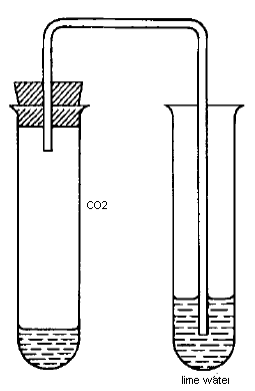
a. With dil HCl:The carbonate salt gives brisk effervescence due to the evolution of CO2 gas
This gas can be tested by passing it through lime water (Ca(OH)2,which turns milky due to the formation of CaCO3 precipitate.
(CO3)2- + 2H+ → CO2↑ + H2O
CO2 + Ca2+ + 2OH- → CaCO3↓ + H2O
Note: Wih prolonged passage of CO2 the turbidity slowly disappears due to the formation of soluble calcium bicarbonate.
CaCO3↓ + CO2 + H2O → Ca2+ + 2HCO3-
b. With Barium chloride solution :The carbonate salt gives a white precipitate of Barium carbonate.This precipitate is soluble in mineral acids.
(CO3)2- + Ba2+ → BaCO3↓
BaCO3 + 2H+ → Ba2+ + CO2↑ + H2O
c. With AgNO3 solution:The carbonate solt gives a white precipitate of silver carbonate This precipitate is soluble in NH3 and in nitric acid. On boiling or on addition of excess reagent, it turns brown due to the formation of Ag2O.
Ag2CO3 + 2H+ → 2Ag+ + CO2↑ + H2O
Ag2CO3 + 4NH3 → 2[Ag(NH3)2]+ + (CO3)2-
Tests for sulphide:
a. With dil HCl :The sulphide salt produces a pungent gas with the smell of rotten eggs(H2S).The gas turns lead acetate paper black due to the formation of black lead sulphide.
S2- + 2H+ → H2S ↑
H2S + Pb2+ → PbS↓
b.With AgNO3 solution:The salt produces a black precipitate of Ag2S which is insoluble in cold but soluble in hot dil HNO3.
S2- + 2Ag+ → Ag2S↓
c.With sodium nitro prusside solution (Na2[Fe(CN)5NO], the sulphide salt gives a violet colouration in the presence of NaOH solution.
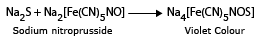
Tests for sulphate :
a.With Lead acetate : In the presence of acetic acid ,the sulphate salt gives a white precipitate of lead sulphate.This precipitate is soluble in excess ammonium acetate on warming.
Na2SO4 + Pb(CH3COO)2 → PbSO4↓ + 2CH3COONa
b. With barium chloride solution:The sulphate salt solution gives a white precipitate of barium sulphate which is soluble only in boiling conc HCl
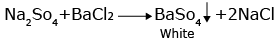
c.With AgNO3 solution: The sulphate salt gives a white crystalline precipitate of silver sulphate
(SO4)2- + 2Ag+ → Ag2SO4↓
Tests for nitrite (NO2 – )
All nitrites are water soluble.Silver nitrite is sparingly soluble.
A nitrite salt with dil H2SO4 gives a brown gas NO2 which turns starch iodide paper blue.
The NO2 gas oxidises the iodide to free iodine which turns starch blue.
NO2- + H + → HNO2
3HNO2 → HNO3 + 2NO ↑ + H2o
2NO ↑ + O2 ↑ → 2NO2 ↑
2KI + 2NO2 → 2KNO2 + I2
I2 + starch → blue colour.
a. Brown ring test: To the nitrite salt solution ,freshly prepared ferrous sulphate solution and dil H2SO4 are added ,Keeping the test tube slanted conc H2SO4 is slowly added along the sides of the test tube.This produces a brown ring at the junction of two layers in the test tube due to the formation of nitroso ferrous sulphate.

b. With cobalt chloride and KCl :
The nitrite salt solution is first acidified with acetic acid . This liberates nitrous acid(HNO2) Cobalt chloride is added along with KCl and warmed .A yellow precipitate appears after some time.The reactions involved are
NaNO2 + CH3COOH → CH3COONa + HNO2
COCl2 + 2NaNO2 → CO(NO2)2 + 2NaCl
2HNO2 → H2O + 2NO + [O]
2Co(NO2)2 + 2NaNO2 + H2O + [O] → 2Co(NO2)3 + 2NaOH
CO(NO2)3 + 3 NaNO2 → Na3[CO(NO2)6] soluble complex
Na3[Co(NO2)6] +3KCl → K3[CO(NO2)6] + 3NaCl yellow precipitate
c. With AgNO3 solution :the nitrite salt solution gives a white precipitate of silver nitrite
NaNO2 + AgNO3 → AgNO2 ↓+ NaNO3
Tests for nitrate(NO3– )
All nitrate salts decompose on treatment with conc sulphuric acid ,liberating reddish brown NO2 gas.
NaNO3 + H2SO4 → NaHSO4 + HNO3
4HNO3 → 2H2O + 4NO2 ↑+ O2 ↑
a.Brown ring test:This is a confirmatory test for the presence of nitrate.The salt solution is acidified with dil H2SO4,then freshly prepared FeSO4 is added followed by addition of a few drops of conc H2SO4.The formation of brown ring confirms the presence of nitrate.
NaNO3 + H2SO4 → NaHSO4 + HNO3
2HNO3 + 6FeSO4 + 3H2SO4 → Fe2(SO4)3 + NO + H2O
FeSO4 + NO → [Fe(NO)]SO4
Tests for chloride(Cl – )
Chlorides react with conc H2SO4 liberating HCl gas .The gas turns moist blue litmus paper red.The presence of the gas can be tested with a glass rod dipped in NH4OH.Dense white fumes of NH4Cl are produced
Cl– + H2SO4 → HCl ↑ + HSO4 –
a. Chromyl chloride test:
The chloride salt is treated with potassium dichromate and conc sulphuric acid and heated.
This produces orange red vapours of chromyl chloride.
These vapours when passed through NaOH solution produce a yellow solution of sodium chromate.
If this solution is neutralised with acetic acid and lead acetate is added,The formation of a yellow precipitate (lead chromate) confirms the presence of chloride.
Note:
Neutralisation is necessary to remove excess NaOH,since lead chromate is soluble in NaOH.
This test is not given by chlorides of Ag,Hg,Pb,Sn and Sb
The reactions involved are:
4NaCl + K2Cr2O7 +6 H2SO4 →4 NaHSO4 + 2KHSO4 + 2CrO2Cl2 ↑+ 3H2O chromyl chloride
CrO2Cl2 + 4NaOH → Na2CrO4 + 2NaCl + 2H2O
Na2CrO4 + (CH3COO)2Pb → PbCrO4↓ +2 CH3COONa yellow precipitate
b. With MnO2 and conc H2SO4 :The chloride salt liberates the greenish yellow gas of chlorine when treated with a pinch of MnO2and a few drops of conc H2SO4.
MnO2 + 2H2SO4 + 2Cl – → Mn2+ + 2SO4 2– + Cl2 ↑ + 2H2O
c. With AgNO3 solution :chlorides give a curdy white porecipitate of AgCl which is insoluble in water and HNO3 but dissolves ammonia due to the formation of a soluble complex salt
Cl – + Ag+ → AgCl ↓
AgCl + 2NH3 → [Ag(NH3)2]Cl
d. With Lead acetate solution : chlorides give a white precipitate od lead chloride.
2Cl – + Pb2+ → PbCl2 ↓
Tests for Bromide (Br – )
Bromide salts react with conc H2SO4 liberating reddish brown fumes of Bromine gas.
2KBr + H2SO4 → K2SO4 +2 HBr↑
2HBr + H2SO4 → 2H2O + SO2 ↑ + Br2↑
Note : H3PO4 cannot be used instead of H2SO4,since the reaction stops after the formation of HBr.
a.With MnO2 and conc H2SO4: The Bromide salt when warmed with MnO2 and conc H2SO4,produces brown vapours of bromine..H2SO4 first converts the bromide to HBr,which then reacts with MnO2 liberating bromine gas
4HBr + MnO2 → MnBr2 + Br2 ↑ + 2H2O
b.With AgNO3 solution : bromide salt solution gives pale yellow precipitate which is partially soluble in ammonia .The salt solution is first acidified with nitric acid before the addition of AgNO3
NaBr+ AgNO3 → NaNO3 + AgBr↓ pale yellow ppt
AgBr + NH3 (aq) → [Ag(NH3)2] Br
c.With lead acetate solution :The bromide salt solution gives a white precipitate of lead bromide PbBr2.
2 Br – + Pb 2+ → PbBr2 ↓
d. With chlorine water : chlorine being more reactive than bromine can displace bromine from the bromide salt.Hence when chlorine water is added to a bromide salt solution bromine gas is evolved and the solution turns orange red.
Addition of excess of clorine water results in the formation of a pale yellow solution of BrCl ,an inter halogen compound.
Cl2 +2 Br – → Br2 + 2Cl –
Br2 + Cl2 → 2BrCl
Note: When an aqueous solution of bromine is shaken with organic solvents like CHCl3, CS2, CCl4,bromine dissolves in them forming a reddish brown solution below the colourless aqueous layer.
e.With potassium dichromate and conc H2SO4: The bromide salt gives vapours of bromine which when passed into water give an orange red solution.
6 KBr + K2Cr2O7 + 7 H2SO4 → 3Br2 + Cr2(SO4)3 + 4K2SO4 + 7H2O
Tests for Iodide (I – )
When the solid iodide salt is warmed with conc H2SO4, violet vapours of Iodine are evolved which turn starch paper blue.The sulphuric acid is reduced to SO2,S and H2S.
a.With AgNO3 solution: The iodide salt solution gives a yellow precipitate of AgI which is insoluble in dil HNO3,slightly soluble in conc ammonia and highly soluble in hypo and KCN due to the formation of soluble complexes.
I – + Ag+ → AgI yellow ppt
AgI + 2CN – → [Ag(CN)2] – + I –
AgI + 2S2O 3 2– → [Ag(S2O3)2]3– + I–
b. With lead acetate: The iodide salt solution gives a yellow precipitate of lead iodide which dissolves in hot water and on sudden cooling gives shiny yellow particles called golden spangles.
2I – + Pb2+ → PbI2 ↓
c.With sodium nitrite solution : The iodide salt solution,liberates iodine in acid medium(CH3COOH or dil sulphuric acid).This test diffrentiates it from chloride and bromide.The iodine produced turns starch paste blue and CCl4 violet
2 I – + 2NO2– + 4 H+ → I2 +2 NO + 2H2O
Characteristic tests of some basic radicals or cations:
Different basic radicals have different solubility products and are precipitated by different reagents.Thus based on the solubility products and the reagents used cations are classified into six groups.
| Group | Radicals | Group reagent | Precipitate |
|---|---|---|---|
| I | Pb2+ | Dil Hcl | Chloride |
| II | Cu2+ | Dil Hcl + H2S | sulphate |
| III | Al3+, Fe3+ | NH4Cl + NH4OH | hydroxide |
| IV | Zn2+, Ni2+ | NH4Cl + NH4OH + H2S | Sulphide |
| V | Ca2+, Ba2+ | NH4Cl + NH4OH + (NH4)2CO3 | Carbonate |
| VI | Mg2+ | ||
| NH4+ |