Redox Reaction of Hydrogen Sulfide and Chlorine
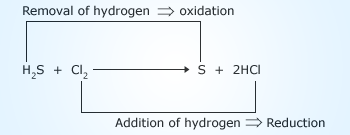 Representation of redox reaction
Oxidation and reduction reactions occur together.
Representation of redox reaction
Oxidation and reduction reactions occur together.
When hydrogen sulfide (H2S) reacts with chlorine (Cl2), sulfur (S) and HCl are formed.
H2S + Cl2  S + 2HCl
S + 2HCl
In this reaction, the H2S is changing into ‘S’ i.e, hydrogen is being removed from hydrogen sulfide. Removal of hydrogen from a substance is known as oxidation. Hence hydrogen sulfide is being oxidized to sulfur.
Further, chlorine (Cl2) is changing to HCl i.e, addition of hydrogen. In other words Cl2 is reduced to HCl. Since oxidation and reduction occur simultaneously, the above is an example of redox reaction.