 Americium and Curium
Americium and Curium
Americium (95Am): This element is prepared by the
bombardment of 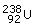 with the
α‐particle which on subsequent emission of neutron and β‐particle will result
in
with the
α‐particle which on subsequent emission of neutron and β‐particle will result
in 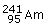
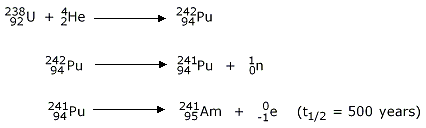
Curium(96Cm): Curium is obtained in similar fashion to
that of Americium but by bombardment of 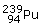 with
α particle.
with
α particle.
 Berkelium and Californium
Berkelium and Californium
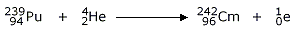
Next two elements are Berkelium (97Bk) and Californium (98Cf). These are produced in the following reactions:
95Am241 + 2He4  97Bk243
+ 2 0n1
97Bk243
+ 2 0n1
Berkelium decays by K electron capture to give Cm.
97Bk242  96Cm243 (by k electron capture)
96Cm243 (by k electron capture)
96Cm242 + 2He4
 98Cf244 + 2
0n1
98Cf244 + 2
0n1
Californium decays by α‐particle emission to give Cm.
98Cf244  96Cm240 + 2He4
96Cm240 + 2He4
Bk has five isotopes with mass equal 243, 244, 245, 249, 250. Longest lived is Bk249 with average life of 1 year.
Cf has eight isotopes with mass equal to 244, 246, 248 to 253. The longest life is 500 years for Cf249.
 Einsteinium
Einsteinium was discovered unexpectedly along with fermium in
debris from the first large hydrogen bomb test, which took place in the Pacific on October 31
1952.
Einsteinium
Einsteinium was discovered unexpectedly along with fermium in
debris from the first large hydrogen bomb test, which took place in the Pacific on October 31
1952.
Einsteinium (99Es): For production of heavier elements, heavier projectiles such as N and O ions have been used in specially designed accelerators. Ex: Element number 99 named Einsteinium (Es) was obtained by the bombardment of U238 with 7N14 ions.
 99Es247 + 5
0n1
99Es247 + 5
0n1
Fermium(100 Fm): Element number 100, named Fermium (100Fm) was discovered in the below reaction–
 100Fm250 +
4
0n1
100Fm250 +
4
0n1
It has a life period of about 3 hrs. Its other isotopes have masses 254, 255 and 256.
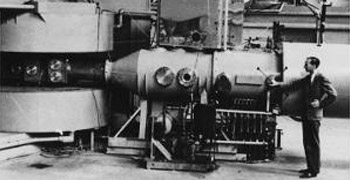 Discovery of Mendelevium
Many transuranium elements including mendelevium were
discovered using the 60-inch
cyclotron at the University of California Lawrence Radiation Laboratory, Berkeley.
Discovery of Mendelevium
Many transuranium elements including mendelevium were
discovered using the 60-inch
cyclotron at the University of California Lawrence Radiation Laboratory, Berkeley.
Mendelevium (100Md): Atomic number of this element is 101. It is prepared by the bombardment of 99Es253 with α‐particles of high energy.
 101Md256 +
0n1
101Md256 +
0n1
Nobelium (102Nb): The atomic number of this element is 102 and is obtained by bombarding 96Cm with 6C12 ions. It has been named as Nobelium (102No) in the honour of Alfred Nobel, inventor of dynamo.
 102No252 + 6
0n1
102No252 + 6
0n1
 Lawrencium
Lawrencium is produced for example by bombarding californium
with boron or americium with oxygen.
Lawrencium
Lawrencium is produced for example by bombarding californium
with boron or americium with oxygen.
Lawrencium (103Lw): Recently element number 103 named Lawrencium (103Lw) has been synthesised by the reaction.
 103Lw253
+ 0n1
103Lw253
+ 0n1
The transactinide elements begin with rutherfordium (atomic number 104). They have only been made artificially, and currently serve no practical purpose because their short half‐lives cause them to decay after a very short time, ranging from a few minutes to just a few milliseconds (except for dubnium, which has a half life of over a day), which also makes them extremely hard to study.