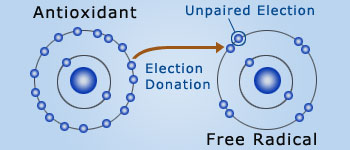
 Free radical theory of aging
The free–radical theory of aging (FRTA) states that organisms age because cells accumulate free radical, damage over time leading to
damage of skin's structural support and decreases its elasticity, resilience, and suppleness.
Free radical theory of aging
The free–radical theory of aging (FRTA) states that organisms age because cells accumulate free radical, damage over time leading to
damage of skin's structural support and decreases its elasticity, resilience, and suppleness.
Sometimes in a chemical reaction of compounds, the constituent elements are not released, but there may be a group of atoms sticking together. These groups are called radicals. Thus a radical is a component of a compound consisting of groups of atoms. Radicals can be positively as well as negatively charged. To simplify the jargons, ions, atoms with a charge, are called simple radicals and groups of atomic radicals are called compound radicals. For example (OH) is called a radical and is negatively charged. Hence it is written as (OH)−, other common compound radicals are (SO4)− −, (NO3)− −, etc.
While dealing with acids, bases and salts, we will constantly deal with ions and radicals, their charges, etc. While writing down chemical equations with the inorganic compounds, in addition to balancing the equations correctly, we also have to deal with balancing the charges on ions and radicals correctly.