
Aldehydes and ketones are identified by their characteristic odor and flavor. The lower aldehydes have sharp pungent odors. As the size of the molecule increases, the odor becomes less pungent and more fragrant. In fact, many naturally occurring aldehydes and ketones are used in the blending of perfumes and flavoring agents.
Methanal is a gas (boiling point &ndash 21°C), and ethanal has a boiling point of + 21°C. That means that ethanal boils at close to room temperature. The other aldehydes and the ketones are liquids, with boiling points rising as the molecules get bigger. The size of the boiling point is governed by the strengths of the intermolecular forces
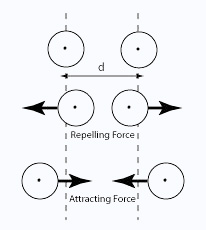
These attractions get stronger as the molecules get longer and have more electrons. That increases the sizes of the temporary dipoles that are set up. This is why the boiling points increase as the number of carbon atoms in the chains increases irrespective of whether you are talking about aldehydes or ketones.
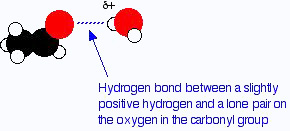
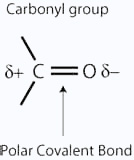
Both aldehydes and ketones are polar molecules because of the presence of the carbon–oxygen double bond. As well as the dispersion forces, there will also be attractions between the permanent dipoles on nearby molecules.
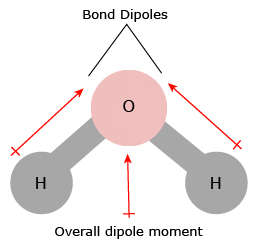
That means that the boiling points will be higher than those of similarly sized hydrocarbons which only have dispersion forces. It is interesting to compare three similarly sized molecules.
| Molecule | Type | Boiling point (°C) |
|---|---|---|
| CH3CH2CH3 | alkane | – 42 |
| CH3CHO | aldehyde | + 21 |
| CH3CH2OH | alcohol | + 78 |
Notice that the aldehyde (with dipole–dipole attractions as well as dispersion forces) has a boiling point higher than the similarly sized alkane which only has dispersion forces. However, the aldehyde's boiling point isn't as high as the alcohol's. In the alcohol, there is hydrogen bonding as well as the other two kinds of intermolecular attraction. Although the aldehydes and ketones are highly polar molecules, they don't have any hydrogen atoms attached directly to
N–Butane 273 b.p.(K) ; 58 Molecular Mass Methoxyethane 281b.p.(K) ; 60 Molecular Mass Propanal 322 b.p.(K) ; 58 Molecular Mass Acetone 329 b.p.(K) ; 58 Molecular Mass Propan–1–ol 370 b.p.(K) ; 60 Molecular Mass Hence increasing order of boiling points of the given compounds is as follows: CH3CH2CH2CH2CH3 < H5C2 – O – C2H5 < CH3CH2CH2CHO < CH3CH2CH2CH2OH
The small aldehydes and ketones are freely soluble in water but solubility falls with chain length.
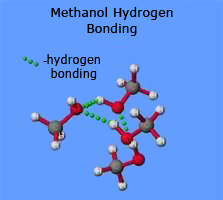
One of the slightly positive hydrogen atoms in a water molecule can be sufficiently attracted to one of the lone pairs on the oxygen atom of an aldehyde or ketone for a hydrogen bond to be formed.
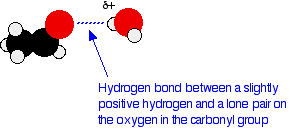
There will also, of course, be dispersion forces and dipole–dipole attractions between the aldehyde or ketone and the water molecules. Forming these attractions releases energy which helps to supply the energy needed to separate the water molecules and aldehyde or ketone molecules from each other before they can mix together. As chain lengths increase, the hydrocarbon "tails" of the molecules (all the hydrocarbon bits apart from the carbonyl group) start to get in the way. By forcing themselves between water molecules, they break the relatively strong hydrogen bonds between water molecules without replacing them by anything as good. This makes the process energetically less profitable, and so solubility decreases.
Aldehydes and ketones are the simplest and most important carbonyl compounds.