 Technetium‐99m : A radioactive tracer
Technetium‐99m : A radioactive tracer
A radio isotope used for diagnosis must emit gamma rays of sufficient energy to escape from the body and it must have a half‐life short enough for it to decay away soon after imaging is completed.
The radioisotope is most widely used in medicine is technetium ‐99m, employed in some 80% of all nuclear medicine procedures ‐ hence some 30 million per year, of which 6‐7 million are in Europe, 15 million in North America, 6‐8 million in Asia/Pacific (particularly Japan), and 0. 5 million in other regions. It is an isotope of the artificially‐produced element technetium and it has almost ideal characteristics for a nuclear medicine scan. These are:
- It has a half‐life of six hours which is long enough to examine metabolic processes yet short enough to minimize the radiation dose to the patient.
- Technetium‐99m decays by a process called "isomeric" which emits gamma rays and low energy electrons. Since there is no high‐energy beta emission the radiation dose to the patient is low.
- The low energy gamma rays it emits easily escape the human body and are accurately detected by a gamma camera. Once again the radiation dose to the patient is minimized.
- The chemistry of technetium is so versatile it can form tracers by being incorporated into a range of biologically‐active substances to ensure that it concentrates in the tissue or organ of interest.
Its logistics also favour its use. Technetium generators, a lead pot enclosing a glass tube containing the radioisotope are supplied to hospitals from the nuclear reactor where the isotopes are made. They contain molybdenum‐99, with a half‐life of 66 hours, which progressively decays to technetium‐99. The Tc‐99 is washed out of the lead pot by saline solution when it is required. After two weeks or less the generator is returned for recharging.
 Myocardial perfusion imaging by Thallium ‐ 201.
Myocardial perfusion imaging by Thallium ‐ 201.
A similar generator system is used to produce rubidium‐82 for PET imaging from strontium‐82 ‐ which has a half‐life of 25 days. Myocardial Perfusion Imaging (MPI) uses thallium‐201 chloride or technetium‐99m and is important for detection and prognosis of coronary artery disease.
Canadian 2006 data shows that there is 56% of Tc‐99 use in myocardial ischemia perfusion, 17% in bone scans, 7% in liver/hepatobiliary, 4% respiratory, 3% renal, 3% thyroid.
For PET imaging, the main radiopharmaceutical is Fluoro‐deoxy glucose (FDG) incorporating F‐18 ‐ with a half‐life of just under two hours, as a tracer. The FDG is readily incorporated into the cell without being broken down, and is a good indicator of cell metabolism.
In diagnostic medicine, there is a strong trend to use more cyclotron‐produced isotopes such as F‐18 as PET and CT/PET become more widely available. However, the procedure needs to be undertaken within two hours reach of a cyclotron, which limits their utility compared with Mo/Tc‐99.
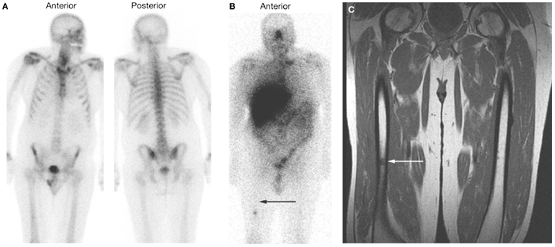 View of bone scans
View of bone scans
- Anterior and posterior view of bone scan.
- Anterior view of 177Lutetium‐J591 scan, which reveals many more lesions than the bone scan, including a discrete lesion in the right femur.
- MRI scan confirming the presence of a lesion in the right femur.
For some medical conditions, it is useful to destroy or weaken malfunctioning cells using radiation. The radioisotope that generates the radiation can be localized in the required organ in the same way it is used for diagnosis ‐ through a radioactive element following its usual biological path, or through the element being attached to a suitable biological compound. In most cases, it is beta radiation which causes the destruction of the damaged cells. This is radionuclide therapy (RNT) or radiotherapy. Short‐range radiotherapy is known as brachytherapy and this is becoming the main means of treatment.
Although radiotherapy is less common than diagnostic use of radioactive material in medicine, it is nevertheless widespread, important and growing. An ideal therapeutic radioisotope is a strong beta emitter with just enough gamma to enable imaging, eg: Lutetium‐177. This is prepared from ytterbium‐176 which is irradiated to become Yb‐177 which decays rapidly to Lu‐177. Yttrium‐90 is used for treatment of cancer, particularly non‐Hodgkin's lymphoma and its more widespread use is envisaged, including for arthritis treatment. Lu‐177 and Y‐90 are becoming the main RNT agents.
Iodine‐131 and phosphorus‐32 are also used for therapy. Iodine‐131 is used to treat the thyroid for cancers and other abnormal conditions such as hyperthyroidism (over‐active thyroid). In a disease called Polycythemia vera, an excess of red blood cells is produced in the bone marrow. Phosphorus‐32 is used to control this excess.
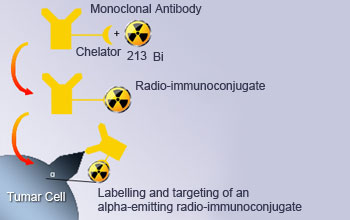 Targeted alpha therapy
Targeted alpha therapy
For Targeted Alpha Therapy (TAT), actinium‐225 is readily available, from which the daughter bismuth‐213 can be obtained (via 3 alpha decays) to label targeting molecules. The bismuth is obtained by elution from an Ac‐225/Bi‐213 generator similar to the Mo‐99/Tc‐99 one. Bi‐213 has a 46‐minute half‐life. The actinium‐225 (half‐life 10 days) is formed from radioactive decay of radium‐225, the decay product of long‐lived thorium‐229, which is obtained from decay of uranium‐233, which is formed from Th‐232 by neutron capture in a nuclear reactor.
Another radionuclide recovered from used nuclear fuel is lead‐212, with half‐life of 10.6 hours, which can be attached to monoclonal antibodies for cancer treatment. Its decay chain includes the short‐lived isotopes bismuth‐212 by beta decay, polonium‐212 by beta decay and thallium‐208 by alpha decay of the bismuth, with further alpha and beta decays respectively to Pb‐208, all over about an hour.
Considerable medical research is being conducted worldwide into the use of radionuclides attached to highly specific biological chemicals such as immunoglobulin molecules (monoclonal antibodies). The eventual tagging of these cells with a therapeutic dose of radiation may lead to the regression ‐ or even cure ‐ of some diseases.
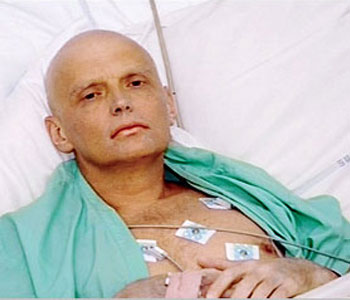 Alexander Litvinenko
Alexander Litvinenko
In 2006 Britain witnessed the apparent murder of one of its newer citizens, a former Russian intelligence official, by poisoning with radioactive polonium. His death was slow and excruciating.
Polonium has about 26 isotopes, all of which are radioactive. Webelements periodic table says that it is 250 billion times more toxic than hydrocyanic acid. It is readily soluble in weak acid. (It was the first element discovered by Marie Curie, in 1898, and named after her native Poland. Her daughter Irene was contaminated with polonium in a laboratory accident and died of leukemia at the age of 59).
Polonium‐210 is the penultimate decay product of U‐238, before it alpha decays to become stable lead. It results from the beta decay of Pb‐210 (in the U‐238 decay series) to Bi‐210 which rapidly beta decays to Po‐210. This gives rise to its occurrence in nature, where uranium is ubiquitous. However, because of its short (138 day) half life, very little Po‐210 would be found in uranium ore or mill tailings ( Webelements suggests 0.1 mg/tonne). Po‐210 levels in soil would be even less, but it is concentrated in tobacco and traces of it can be found in smokers urine.
Po‐210 can also be made by neutron irradiation of Bi‐209, and that is most likely source of any significant quantity. Russia has used Po‐210 as a heat source in short‐life spacecraft and lunar rovers. It also operates reactors using lead‐bismuth cooling, which becomes contaminated with Po‐210 due to neutron bombardment.
Because its half‐life is so short, a gram of Po‐210 is about 5000 times as radioactive as a gram of radium ‐ which sets the standard of activity. But at 138 days its half life is long enough for it to be manufactured, transported and administered before its loses its potency. It would not put the carrier at much risk, since alpha radiation is only really a hazard inside the body ‐ a layer of skin is protection. About 10 micrograms (2 GBq) was said to have been used, administered in a cup of tea (it would be warm due to the decay).
However, simply dosing someone with polonium might not have much effect if it simply went in one end and out the other in a day or two without being absorbed from the gut. It would probably need to be complexed on to an organic carrier which would enter the bloodstream and take it to vital organs where it would stay. (This is what happens with targeted alpha therapy (TAT) using very low levels of alpha‐active radioisotopes: the carriers take them to dispersed cancerous tissues where they are needed).