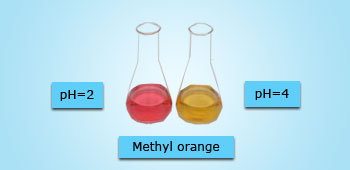
 Colors of Methyl Orange Indicator in different pH Solutions
Methyl Orange is used in a Titration as an indicator. This is because it is densely colored and give a distinct color change. It is mostly preferred in titration of weak bases with strong acids. It changes from red to yellow depending with the strength of the reactants.
Colors of Methyl Orange Indicator in different pH Solutions
Methyl Orange is used in a Titration as an indicator. This is because it is densely colored and give a distinct color change. It is mostly preferred in titration of weak bases with strong acids. It changes from red to yellow depending with the strength of the reactants.
We can see the color changes of methyl orange because it absorbs light in the visible part of the electromagnetic spectrum. Its molecule contains an extended system of delocalized electrons called a chromophore.
The differences in energy between the quantized electronic energy levels correspond to the energies of photons of visible light. Electrons are promoted when these photons are absorbed, removing their frequencies from those that enter the eye. In methyl orange, when the molecule becomes protonated in acidic solution, the differences in energy between the electron energy levels change slightly from the un–protonated form. This results in the absorption of different frequencies of visible light and so a change in color of the indicator.
In alkaline solution it absorbs blue–green and red light making it appears yellow. Add acid Methyl orange in acidic solution absorbs blue–green light, which makes its solution appear red. Add base for phenolphthalein: pH 8.2 = colorless; pH 10 = red; for bromophenol blue: pH 3 = yellow; pH 4.6 = blue