 Since metallic bonds involve loosely-bound electrons, there can be a
relatively large number of probable orientations for a metal bond. In other words, the bonds are weak and
non-directional. This characterizes metals as tending to crystallize in relatively close-packed structures
with a large number of nearest neighbours.
Since metallic bonds involve loosely-bound electrons, there can be a
relatively large number of probable orientations for a metal bond. In other words, the bonds are weak and
non-directional. This characterizes metals as tending to crystallize in relatively close-packed structures
with a large number of nearest neighbours.
Metals are all good conductors of heat and electricity. Hence you find them in electric cables, light bulbs, circuits and cooking pans. For example, Cu, Al, and Ag are very good conductors of electricity; comparatively, lead (Pb) is not such a good conductor of electricity. Most common metals like tin, copper, and chromium do not dissolve or react with water. This is the reason why we use such metals in making pots, and pans for cooking and why drinks and foods are sold in tin cans.
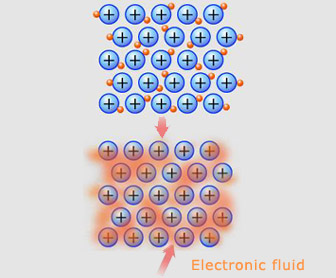
Metallic atoms are held by electrostatic forces, joined in a particular way that makes them what they are. The metallic properties of a given metal, depend on the behaviour of the atoms of the metallic elements. The outer electrons of most metal atoms tend to be weakly held to the atomic nucleus.
Consequently, these outer electrons are easily dislodged, leaving behind positively charged metal ions. The electrons dislodged from a large group of metal atoms flow freely through the resulting metal ion grid. This "fluid" of electrons holds the positively charged metal ions together in a type of chemical bond known as a metallic bond.