Homoleptic Carbonyl
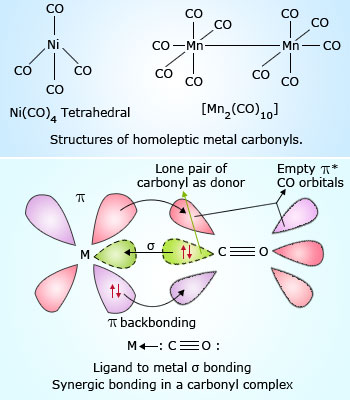
Compounds in which a metal is bonded with only carbonyl ligands are called Homoleptic Carbonyl compounds. For example, tetracarbonylnickle(0), pentacarbonyliron(0), decacarbonyldimanganese(0) etc.
Decacarbonyldimanganese(0) is made up of two square pyramidal Mn(CO)5 units joined by a Mn-Mn bond. In these complexes, the metal-carbon bond possess both σ and π characters.
M-C σ bond is formed by donating lone pair of electrons to a vacant orbital of metal, while,
M-C π bond is formed by the donation of a pair of electrons from a filled d orbital of metal to the vacant anti-bonding π⋆ orbital of CO. These bonds strengthens the linkage between metal and ligand and are known as Synergic bonding