Ionic product of water
- Pure water is a weak electrolyte and undergoes self-ionistion to a small extent.
- "The product of concentrations of H+ and OH– ions in water at particular temperature is known as ionic product of water". It is designed as Kw.
- H2O ⇌ H+ + OH–; ΔH = +57.3 kJM–1
-
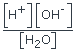 ; K[H2O] = [H+][OH–]; Kw = [H+][OH–]
; K[H2O] = [H+][OH–]; Kw = [H+][OH–] - The value of Kw increases with increase of temperature, i.e., the concentration of H+ and OH– ions increases in temperature.
- The value of Kw at 25°C is 1×10–14 mole/liter. Since pure water is natural in nature, H+ ions concentration must be equal to OH– ion concentration.
- [H+] = [OH–] = x or [ H+][OH–] = x2 = 1×1014 or x = 1×10–7M or [H+] = [OH–] = 1×10–7 mole liter–1
- This shows that 25°C, in liter only 1010 mole of water is in ionic form out of a total of approximately 55.5 moles.
- Thus when, [H+] = [OH–]; the solution is natural
- [H+] > [OH–]; the solution is acidic
- [H+] < [OH–]; the solution is basic