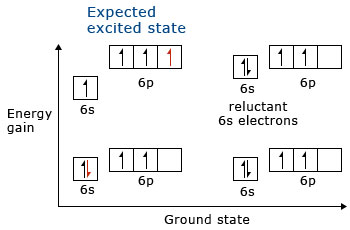
Inert Pair Effect
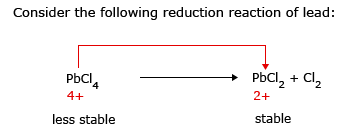
This can be explained as follows:
Electron configuration of lead is written as

The two 6s electrons in the valence shell are reluctant to participate in bond. This is due to increased effective nuclear charge of poorly shielded electrons in complex shaped d orbital.