HydrolyzedChemical Reactions of Aldehydes and Ketones
Reduction to alcohols: Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4) as well as by catalytic hydrogenation. Their structures are:

The reduction of an aldehyde The same organic product is obtained either by using lithium tetrahydridoaluminate or sodium tetrahydridoborate. Example, with ethanal you get ethanol:

In general terms, reduction of an aldehyde leads to a primary alcohol. Mechanism: Using lithium tetrahydridoaluminate (lithium aluminum hydride) In the first stage, a salt is formed containing a complex aluminum ion. The following equations show what happens if you start with a general aldehyde or ketone. R and R' can be any combination of hydrogen or alkyl groups.
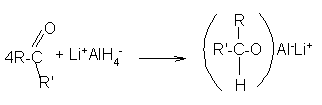
The product is then treated with a dilute acid (such as dilute sulphuric acid or dilute hydrochloric acid) to release the alcohol from the complex ion.
The alcohol formed can be recovered from the mixture by fractional distillation.

Fractional distillation
The reduction of a ketone: Again the product is the same whichever of the two reducing agents you use. Example, with propanone you get propan−2−ol:
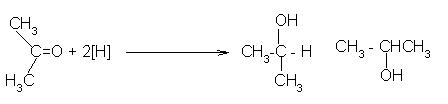
Reduction of a ketone leads to a secondary alcohol.
- Using sodium tetrahydridoborate (sodium borohydride) Solid sodium tetrahydridoborate is added to a solution of the aldehyde or ketone in an alcohol such as methanol, ethanol or propan−2−ol. Depending on which recipe , it is either heated under reflux or left for some time around room temperature. This almost certainly varies depending on the nature of the aldehyde or ketone. At the end of this time, a complex similar to the previous one is formed.In the second stage of the reaction, water is added and the mixture is boiled to release the alcohol from the complex.
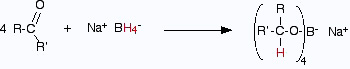 Again, the alcohol formed can be recovered from the mixture by fractional distillation.
Again, the alcohol formed can be recovered from the mixture by fractional distillation.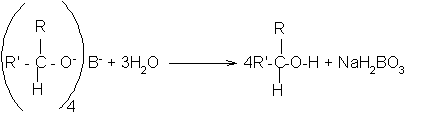
- Reduction to hydrocarbons: The carbonyl group of aldehydes and ketones is reduced to CH2 group.
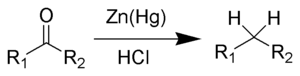
- Clemmensen reduction is a chemical reaction described as a
reduction of ketones (or aldehydes) to alkanes using zinc amalgam and
hydrochloric acid. This reaction is named after Erik Christian Clemmensen, a
Danish chemist.
The Clemmensen reduction is particularly effective at reducing aryl−alkyl ketones. With aliphatic or cyclic ketones, zinc metal reduction is much more effective. The substrate must be stable in the strongly acidic conditions of the Clemmensen reduction. As a result of Clemmensen Reduction, the carbon of the carbonyl group involved is converted from sp2 hybridization to sp3 hybridization. The oxygen atom is lost in the form of one molecule of water. Acid sensitive substrates should be reacted in the Wolff−Kishner reduction, which utilizes strongly basic conditions.
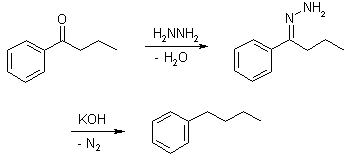
- Wolff−kishner reduction The reduction of aldehydes and ketones to alkanes. In Wolff−kishner reaction Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
Hydrazine
Wolff−Kishner Reduction
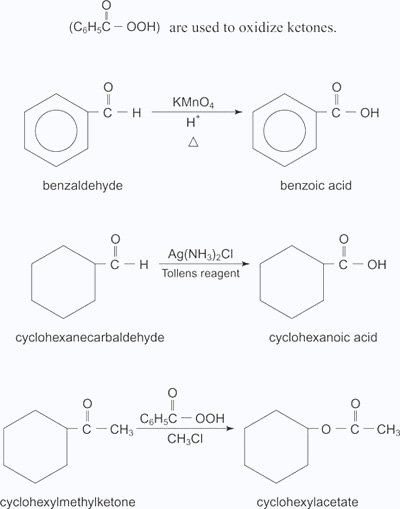
Oxidations of aldehydes and ketones: Aldehydes can be oxidized to carboxylic acid with both mild and strong oxidizing agents. However, ketones can be oxidized to various types of compounds only by using extremely strong oxidizing agents. Ketones are generally oxidized under vigorous conditions, i.e.,strong oxidizing agents and at elevated temperatures. Their oxidation involves carbon−carbon bond cleavage to afford a mixture of carboxylic acids having lesser number of carbon atoms than the parent ketone. Typical oxidizing agents for aldehydes include either potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7) in acid solution and Tollens reagent. Peroxy acids, such as peroxybenzoic acid are used to oxidize ketones.
Example:
- Benzaldehyde is oxidized to benzoic acid by KMnO4 oxidizing agent.
- Cyclohexane carbaldehyde is oxidized to Cyclohexanoic acid by Tollen's reagent.
- Cyclohexyl methyl ketone is oxidized to cyclohexylacetate by using peroxybenzoic acid as oxidizing agent.
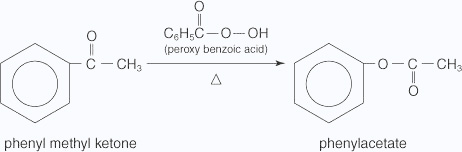
Baeyer–Villiger oxidation is a ketone oxidation, and it requires the extremely strong oxidizing agent peroxybenzoic acid. For example, peroxybenzoic acid oxidizes phenyl methyl ketone to phenyl acetate (an ester).

Haloform reaction: In haloform reaction Aldehydes and ketones having one methyl group linked to a carbonyl carbon atom (i.e )methyl ketone are oxidized by sodium hypohalite to sodium salts of corresponding carboxylic acids having 1 carbon atom less than that of carbonyl compound.
Sodium hypochloride
The methyl group is converted to haloform.
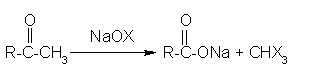
Other reactions:
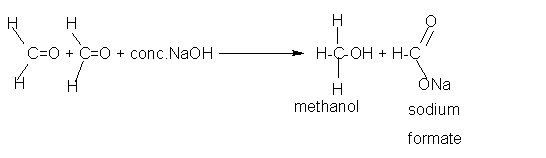
Cannizzaro reaction: Aldehydes which do not have α hydrogen atom react with concentrated sodium hydroxide (NaOH) or potassium hydroxide (KOH) in such a way that one molecule get oxidized to acid and the second molecule gets reduced to alcohol. Note two molecules of aldehyde participates in the reaction. This self oxidation−reduction under the influence of a base is known as the Cannizzaro's reaction.
Example:
- Formaldehyde does not possess a−hydrogen atom and therefore undergoes
Cannizzaro's reaction. Acetaldehyde does not give this reaction.
Formaldehyde(HCHO) two molecules + warm NaOH give Methanol (CH3OH) and Sodium Formate (CHOONa) CH3OH is the reduction product HCHO becomes CH3OH (two hydrogen atoms are getting in). CHOONa is the oxidation product. one 'H' has gone out and One 'O' came in along with Na.
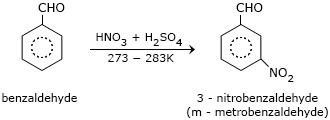
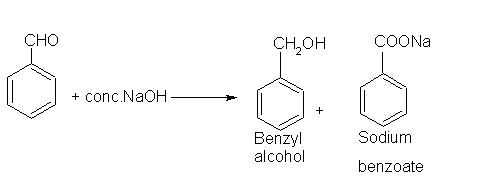
- Benzaldehyde
Electrophilic substitution reaction: Aromatic aldehydes and ketones undergo electrophilic substitution at the ring in which the carbonyl group acts as a deactivating and meta−directing group.