Electrovalency of more elements
Valency of Oxygen:
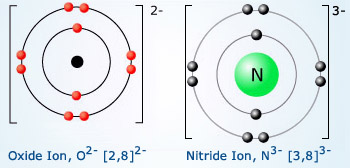 Valency of Oxygen and Nitrogen Oxygen and nitrogen
require 2 and 3 electrons respectively to attain the nearest stable gas configuration. Thus, oxygen gains
two electrons from donating species such as Mg. Similarly nitrogen accepts 3 electrons from donors making
its valency as 3.
Valency of Oxygen and Nitrogen Oxygen and nitrogen
require 2 and 3 electrons respectively to attain the nearest stable gas configuration. Thus, oxygen gains
two electrons from donating species such as Mg. Similarly nitrogen accepts 3 electrons from donors making
its valency as 3.
The atomic number of oxygen is 8, so its electronic configuration is 2, 6. The oxygen atom has 6 valence electrons, so it needs 2 more electrons to complete the 8‐electron structure. The oxygen atom gains 2 electrons to form an oxide ion O−2, with electrovalency of − 2.
Valency of Nitrogen:
The atomic number of nitrogen is 7, so its electronic configuration is 2, 5. It needs 3 more electrons to complete the 8‐electron structure. The nitrogen atom gains 3 electrons to form a nitride ion, N−3. So electrovalency of nitrogen is −3. What is the electrovalency phosphorous whose atomic number is 15?