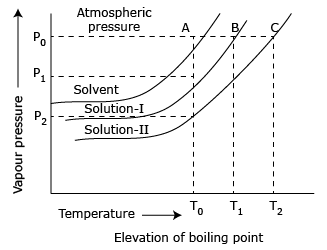
Determination of boiling point
Elevation in boiling point is determined by Landsberger's method and Cottrell's method. Study of
elevation in boiling point of a liquid in which a
non–volatile solute is dissolved is called as ebullioscopy. Hence the constant
Kb is called Ebullioscopic constant
Important relation concerning elevation in boiling
point.
(1) Depression in freezing point is directly proportional to the lowering of vapour
pressure. ΔTf ∝ P0–P
(2) ΔTf = Kf × m
where Kb = molal elevation constant or ebullioscopic constant of the
solvent; m = Molality of the solution, i.e., number of moles of solute per 1000g of the
solvent; ΔTb = Elevation in boiling point
(3) 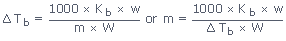
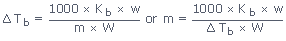
where, Kb is molal elevation constant and defined as the elevation in b.pt. produced when
one mole of the solute is dissolved in 1 kg of the solvent
w and W are the weights of solute and solvent and m is the molecular weight of the solute.
w and W are the weights of solute and solvent and m is the molecular weight of the solute.
(4) 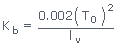
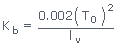
where T0 = Normal boiling point of the pure solvent; lV = Latent
heat of evaporation in cal/g of pure solvent; Kb for water is 0.52k–kg
mol–1.
Azeotropic mixture :
Azeotropics are defined
as the mixtures of liquids which boils at constant temperature like a pure liquid and
possess same composition of components in liquid as well as in vapour phase. Azeotropes
changes into vapour state at constant temperature and there components cannot be separated
by fractional distillation. Azeotropes are of two types as described below
(1) Minimum boiling azeotrope :
For the
solution with positive deviation there is an intermediate composition for which the vapour
pressure of the solution is mixture and hence, boiling point is minimum. At this composition
the solution distils at constant temperature without change in composition. This type of
solutions are called minimum boiling azeotrope. e.g.,
H2O + C2H5OH,H2O +
C2H5CH2OH
CHCL3 + C2H5OH, (CH3)2CO +
CS2
(2) Maximum boiling azeotrope :
For the
solutions with negative deviations there is an intermediate composition for which the vapour
pressure of the solution is minimum and hence, boiling point is maximum. At this composition
the solution distils at constant temperature without the change in composition. This type of
solution are called maximum boiling azeotrope. e.g.,
H2O + HCl,H2O + HNO3,H2O +
HClO4
Some azeotropic mixtures
| Mixture | % composition of azeotrope | Boiling point(pressure = 1 atm) |
|---|---|---|
| Nitric acid-Water | 68% Nitric acid | 125.5°C |
| Acetic acid-Pyridine | 65%Pyridine | 139.0°C |
| Chloroform-Acetone | 80% Pyridine | 65.0°C |
| Hydrogen chloride-Water | 79.8% Water | 108.6°C |
Examples :
Ex1:
The elevation in boiling point of asolution of 13.44 g
of
CuCl2 in 1 kg of water using the following information will be :
(Molecular weight of CuCl2 = 134.4 and Kb = 0.52 K
molal–1)
Sol:
CuCl2 is an electrolyte which ionise in solution as
follows:
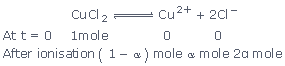
Thus, number of particles after ionisation
= 1 – α + α + 2α = 1 + 2α.
∴ van't Hoff factor (i)
= (Number of particles after ionisation / Number of particles before ionisation)
or (i) = [(1 + 2α) / 1] (On 100% ionisation α = 1)
= [(1 + 2 × 1) / 1] = 3
The elevation in boiling point (when colligative property is abnormal)
δTb = i × Kb × m
m → Molality of solution
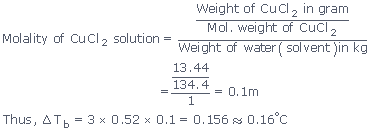
Ex2:
Ratio of ΔTb/Kb of 6%
AB2 and 9% A2B (AB2 and A2B both are
non – electrolytes is 1 mol/kg in both cases. Hence, atomic masses of A and B are
respectively:
Sol:
ΔT =
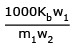
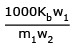
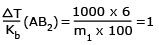
∴ m1 (A2B) = 60 = A + 2B
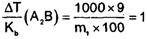
∴ m1 (A2B) = 90 = 2A + B
A = 40, B = 10