Cryoscopic constants

Depression in freezing point is determined by Beckmann's method and Rast's
camphor method. study of depression in freezing point of a liquid in which a
non–volatile solute is dissolved in it is called as cryoscopy.
Important relations concerning depression in freezing
point
(1) Depression in freezing point is directly proportional to
the lowering of vapour pressure. ΔTf ∝ P0–P
(2) ΔTf = Kf × m
where Kf = molal depression constant or cryoscopic constant; m = Molality of
the solution, (i.e., number of moles of solute per 1000g of the solvent);
ΔTf = Depression in freezing point
(3) 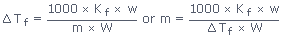
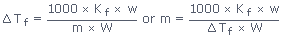
where Kf is molal depression constant and defined as the depression in freezing
point produced when 1 mole of the solute is dissolved in 1kg of the solvent.w and W are the
weights of solute and solvent respectively and m is the molecular weight of the solute
(4)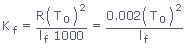
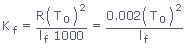
where T0 = Normal freezing point of the solvent; lf = Latent heat
of fusion/g of solvent; Kf for water is 1.86k–kg
mol–1
Q.
The freezing point of solution containing 50
cm3 of ethylene glycol in 50g of water is – 34°C Assuming ideal
behaviour, the density
of ethylene glycol in g/cm3 is:
Sol:
V = 50 cm3 (for ethylene glycol)
Let density of ethylene glycol be d
Wt of ethylene glycol (w1) = 50 × d
Wt. of water W2 = 50g.
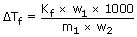
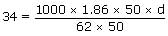
∴ d = 1.133g/cm3.
Q.
The amount of ice that will separate out on cooling a
solution containing 50g of ethylene glycol in 200g of water to – 9.3°C
(Kf
for water = 1.86 Kmol– 1Kg)
Sol:
ΔTf =
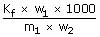
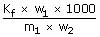
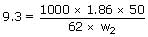
W2 = 161.2g
Wt. of ice separated = (200 – 161.2) g = 38.71g.