 Covalency of Nitrogen
Nitrogen has 5 electrons in outermost shell, it shares 3 electrons from other atoms to attain the octet configuration.Hence the covalency of N is 3.
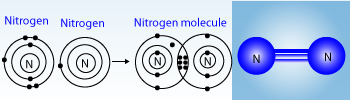
Covalency of Nitrogen
Nitrogen has 5 electrons in outermost shell, it shares 3 electrons from other atoms to attain the octet configuration.Hence the covalency of N is 3.
Covalency of Nitrogen : A nitrogen atom has 5 valence electrons. So it can share its three electrons with three electrons of another atom to attain the 8‐electron inert gas electronic configuration. The covalency of nitrogen is 3. For example, in the formation of a nitrogen molecule, N2, each nitrogen atom shares its three electrons with the other atom. So the valency of nitrogen in the N2 molecule is 3.
Covalency of Carbon :The atomic number of carbon is 6. So its electronic configuration is 2, 4. It requires 4 more electrons to complete the "octet". It gets these electrons by sharing. Therefore, the covalency of carbon is 4. For example, in the formation of a methane molecule, CH4, the carbon atom shares its 4 electrons with four electrons of four hydrogen atoms. So the valency of carbon in CH4 molecule is 4.