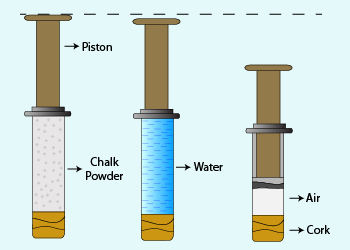
To examine the compressability among the three states, chalk powder (a soft solid) and water (a liquid) are filled in a syringe. It was difficult to push the piston in case of chalk powder when compared with water. Finally the experiment is repeated with air (a gaseous mixture). The piston worked to maximum extent inferring an easy compressability of gases.
Reason:
Particles in gases move around due to lot of space between them. On applying force by means of a piston, they come closer (compress) easily. As the distance between the particles of solids is very less, they could not be compressed further. Similar experience with the particles of liquid is observed.