Raoult's law ‐ Relative lowering of vapour pressure
Relative lowering of vapour pressure is defined as the ratio of lowering
of vapour pressure to the vapour pressure of the pure solvent. It is determined by
Ostwald–Walker method
Thus according to Raoult's law,
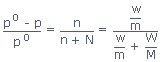
where, p = Vapour pressure of the solution
p0 = Vapour pressure of the pure solvent
n = Number of moles of solute
N = Number of moles of the solvent
w and m = weight and mol.wt of solute
W and M = weight and mol.wt. of the solvent.
Ex 1:
The v.p. of a solution containing 2 g of nonvolatile solute in 78 g of benzene at a certain temperature is 195 mm Hg. (v.p. of benzene at that temperature is 200 mm Hg). Molecular weight of the solute is
Sol:
(p0 – p) / p0 = (w
/ m) ×(M / W).
Given values are
p0 = 200
p = 195
m1 = ?,
w1 = 2grams, M1 = 78, W1 = 78 grams
substituting the
values
(200 - 195)/200 = (m1 × 78)/(2 × 78)
∴ m = (2 × 200)/5 = 80.
∴ m = (2 × 200)/5 = 80.
Ex 2:
The wt. of solute (M = 60) required to be added in (80g of water to reduce the vapour pressure 4/5th of pure water is
Sol:
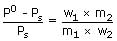
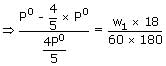
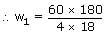 = 150g
= 150g