 Elements in the Human Body
Elements in the Human Body
Life is composed of a myriad of biomolecules (molecules found in living organisms). With all of life's diversity, however, there is a ubiquitous presence of carbon, oxygen, and hydrogen. Carbon plays a particularly important role in these molecules by virtue of its unique chemical properties.
As our knowledge of the chemistry of living systems (biochemistry) increases, we learn more about essential elements. Mammals like ourselves are thought to use only 25 of the 116 known elements. Apart from oxygen, these elements are not found as ‘pure’ elements. Instead, they are found either dissolved in water in an ionic form, such as Sodium ions and chloride ions, or as parts of large molecules, such as hemoglobin.
Most of the human body is made up of water, with cells consisting of 65−90% water by weight. Therefore, it isn't surprising that most of a human body's mass is oxygen. Carbon, the basic unit for organic molecules, comes in second. 99% of the mass of the human body is made up of just six elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus.
Scientists believe that about 25 of the known elements are essential to life. Just four of these − carbon (C), oxygen (O), hydrogen (H) and nitrogen (N) − make up about 96% of the human body.
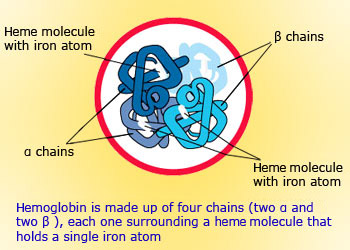 Structure of Hemoglobin
Structure of Hemoglobin
Although many of the elements are required in very small amounts, they do play a very important role in keeping the body working effectively:
- Much of the 3−4 grams of iron in the body is found in hemoglobin, the substance responsible for carrying oxygen from the lungs to the rest of the body.
- The body has about 75 mg of copper, about one−third of which is found in the muscles. Copper combines with certain proteins to produce enzymes that act as catalysts to help a number of body functions. Some are involved in the transformation of melanin for pigmentation of the skin, and others help to form cross−links in collagen and elastin and thereby maintain and repair connective tissues. This is especially important for the heart and arteries. Research suggests that copper deficiency is one factor leading to an increased risk of developing coronary heart disease. A well balanced diet will provide the working body with all of its essential trace elements.
 Hyperactivity in children
Hyperactivity in children
Large amounts of essential elements can prove toxic:
- Too much copper in the diet can result in damage to the liver, discoloration of the skin and hair, and can cause hyperactivity in children.
- Too much iron in the diet can result in damage to the heart and liver. Too little of any given essential element can result in ill health and, if left untreated, could result in death.
- Zinc is a component of certain digestive enzymes and other proteins. Low amounts in the diet can result in growth failure, scaly skin inflammation, reproductive failure and impaired immunity.
- People who suffer from iron deficiency show symptoms such as lack of energy, getting tired easily and being short of breath.