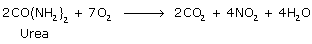BURNING OF ELEMENTS
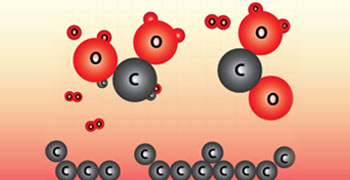
When elements are burnt in excess of air, their oxides are formed.
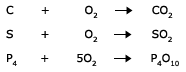
Suppose an element has the capacity to form more than one oxide. In that case, the lower oxide is usually formed when the air supply is insufficient.

All compounds containing two or more combustible elements can burn to give their oxides as final products

All organic compounds can burn in the air as they contain carbon and hydrogen.
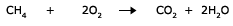
Besides, some lower oxides also burn to give the corresponding higher oxides.
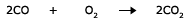
Compounds containing carbon, hydrogen and oxygen also burn to give carbon dioxide and water.
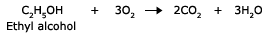
Any organic compound on burning in the air forms the oxides of carbon, hydrogen and other elements.