Hydrolyzed Isomerism
Following types of isomerism is possible in aldehydes and ketones.
Chain isomerism:
The isomers which differ in the length of carbon chain are called chain isomers. Aldehydes which
contain four or more carbon atoms, and the ketones which contain five or more carbon atoms show chain
isomerism.
Example:
- Butanal (n – butyral–dehyde) and methylpropan–2–al
(iso–butyraldehyde) are chain isomers.
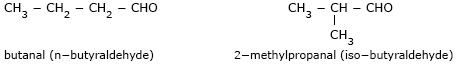
- Pentan–2–one (methyl propyl ketone) and
3–methylbutan–2–one (methyl isopropyl ketone) are chain isomers.
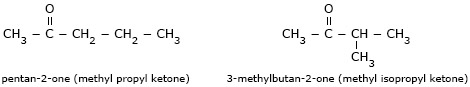
Position isomerism:
The isomers having carbonyl group at different locations in the chain are called positional isomers.
Example:
Pentanone can have carbonyl group at two different locations as shown below.
Aliphatic aldehydes:
Do not show position isomerism, because the – CHO group is always present at the end of the chain.