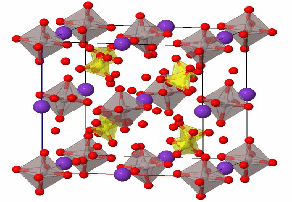
Alum is the common name for the hydrated salt of potassium aluminum sulfate. This is called a "double salt" because it contains two different cations – potassium and aluminum t crystallize together in a single solid. The alum crystal structure(fig.) contains aluminum (gray) and potassium (purple) ions, as well as tetrahedral sulfate 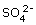 counter ions.
counter ions.
The crystal is a hydrate, and the red atoms shown attached to the metals indicateoxygens from the water molecules. (Hydrogens are not shown, for simplicity.)
As indicated in the figure, the metal atoms have an octahedral coordination sphere, with a single metal ion surrounded by 6 water molecules.
The formula for this compound is KAl[SO4]2.12H2O.
It is important to note that this compound is a salt, and so it fully dissolves into individual ions in solution.
Here, the octahedral coordination of the metal ions depends on the crystal structure, unlike coordination compounds that maintain the same coordination geometry in the solid and solution states.