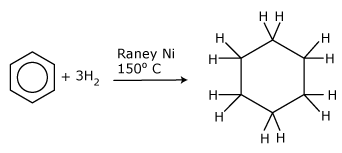
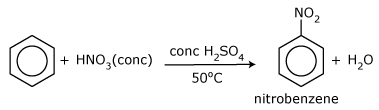Reactions with hydrogen
The alkenes undergo rapid addition reactions with hydrogen in the presence of a nickel catalyst. It was the fact that benzene does not readily undergo these sort of addition reactions that pointed to its true structure. When benzene is mixed with hydrogen it needs a Raney nickel catalyst (this is finely divided to give it a very large surface area and thus very high catalytic activity) as well as a temperature of around 150°C before it will undergo addition reactions. Benzene then reacts with hydrogen to form cyclohexane – there are no unsaturated intermediates. If one mole of hydrogen reacts with one mole of benzene, one–third of the benzene forms cyclohexane and the rest remains as benzene. Two moles of hydrogen convert two–thirds of the benzene to cyclohexane, and it takes three moles of hydrogen to give a complete hydrogenation of one mole of benzene to cyclohexane. The formation of cyclohexane from benzene, shown in the figure, is an important industrial process as it leads to the production of nylon.
Reactions with acidified potassium manganate (VII)
We know that both the alkenes and the alkynes react with acidified potassium manganate(VII), which is a strong oxidizing agent. Benzene, however, does not react at all.
Reactions with nitric acid – nitration
Benzene will react with nitric acid, but only with a mixture of concentrated nitric acid and concentrated sulphuric acid (known as nitrating mixture) at 50°C. The NO2 group is substituted for one of the hydrogen atoms of the benzene ring, as shown in the below figure, forming a yellow oily substance, which is nitrobenzene. This is another electrophilic substitution reaction of the benzene ring. Concentrated nitric acid alone reacts only very slowly with benzene at 50°C, and at that temperature sulphuric acid does not react at all. When mixed, the two acids appear to react to form an electrophile that can attack the benzene. The electrophile is the nitronium cation, NO2+.