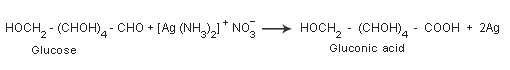Other Properties Of Glucose
Glucose adds a molecule of hydrogen cyanide to give a cyanohydrin.
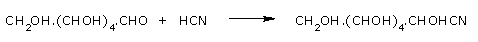
These reactions confirm the presence of a carbonyl group in glucose. Glucose reduces ammoniacal silver nitrate solution (Tollens reagent) to metallic silver and also Fehlings solution to reddish brown cuprous oxide and itself gets oxidized to gluconic acid.
This confirms the presence of an aldehydic group in glucose.