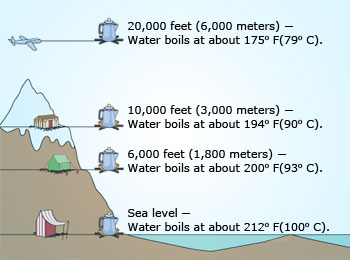
The boiling point is the temperature at which a liquid is converted to a gas. As the temperature of the water increases, its vapor pressure increases. When the vapor pressure equals the atmospheric pressure on the liquid, the liquid will boil. At high altitudes, the boiling point of liquids is lower than at sea level.
For water, the vapor pressure reaches the standard sea level atmospheric pressure of 760 mm Hg at 100°C. Since the vapor pressure increases with temperature, it follows that for pressure greater than 760 mm Hg (e.g., in a pressure cooker), the boiling point is above 100°C and for pressure less than 760 mm Hg (e.g., at altitudes above sea level), the boiling point will be lower than 100°C. As long as a vessel of water is boiling at 760 mm Hg, it will remain at 100°C until the phase change is complete.
We know that when a liquid boils, the air bubbles appear at the bottom of the container and rise to the surface. Bubbles in the liquid can form and sustain only when the pressure of the vapor within the bubbles is great enough to resist the pressure of the surrounding liquid. At boiling point, 100°C for water at atmospheric pressure, molecules are energetic enough to exert a vapor pressure as great as the pressure of the surrounding water.
Either by going deeper below the surface of the liquid or by increasing the air pressure above the liquid surface, extra pressure can be applied. On applying extra pressure, the molecules in the vapor must move faster to exert enough pressure to keep the bubble from collapsing. A pressure cooker works on the same principle. As shown in the figure when we heat the contents in the sealed pressure cooker, the evaporating vapour builds up a pressure on the surface of the liquid, which at first prevents boiling. Bubbles that would normally form are crushed.
If you still heat it then the temperature is raised to 100°C. Boiling occurs only when the vapor pressure within the bubbles overcomes the increased pressure on the water. The boiling point is raised. Hence we can say that the boiling point of the liquid depends not only on temperature but also on pressure as well.
At high altitudes, water boils at a lower temperature. For example, in Pune, India, which is at an altitude of about 400 meters from mean sea level, the boiling point of water is 98°C only. If the temperature of the boiling water is too low, food will not cook at all. It is important to note that it is the high temperature of the water that cooks the food, not the boiling process itself. In fact at high altitude regions, a pressure cooker is must for cooking food quickly and efficiently.
When atmospheric pressure increases (at sea level), the boiling point becomes higher, and when atmospheric pressure decreases at increasing heights above sea level, the boiling point becomes lower.
- High-pressure steam rapidly transfers heat to the surface of any food not submerged in liquid.
- A spring-loaded valve is normally open so that air can escape. As heating begins, expanding vapor pushes this valve up, closing off the vent. The valve regulates the pressure inside the cooker to a preset level: typically 0.7 or 1 bar above atmospheric pressure. At these elevated pressures, water boils at 114°C or 121°C.
- The sealing ring, typically a rubber gasket, prevents steam and air from escaping as they expand. This causes the pressure in the vessel to build as the temperature rises. Any food particles stuck in the seal can cause it to leak steam, so check and clean the gasket regularly.
| Evaporation | Boiling |
|---|---|
| 1. It is a slow process. It happens continuously on the liquid surface. | It is a rapid process. It happens only when the right amount of heat is supplied to the entire liquid. |
| 2. It takes place at all temperatures. | For a given pressure, boiling takes place at a fixed boiling temperature only. |
| 3. For a given liquid, the rate of evaporation depends on the surface area exposed. |
For a given liquid, the rate of boiling depends on the pressure to which the
liquid
is subjected. Example: water in a pressure cooker. |
|
4. Some liquids that evaporate readily are called volatile liquids. Example: Alcohol, Ether. |
Liquids can be made to boil at different temperatures by changing the
pressure. Example: water in a pressure cooker. Most of the domestic pressure cookers have an operating pressure of 11.6 – 15 psi. At this pressure, water boils at 121°C. The same liquid (water) boils at 100°C in an open system (at atmospheric pressure: 14.7 psi). |