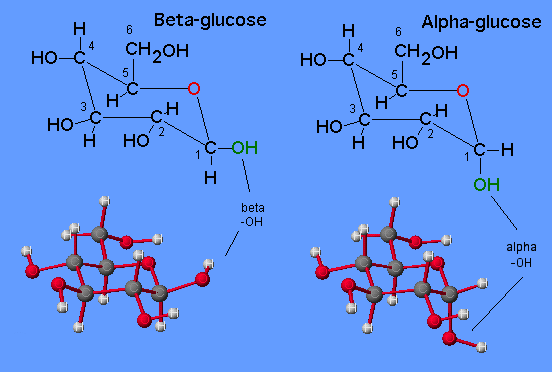
 Structures of β and α glucose
In alpha glucose, the hydroxyl group is attached facing down
and away from the main structure, while in the beta glucose, the hydroxyl group is attached
above the ring and on the first carbon. Alpha glucose and beta glucose are isomers and contain
the same number of atoms.
Structures of β and α glucose
In alpha glucose, the hydroxyl group is attached facing down
and away from the main structure, while in the beta glucose, the hydroxyl group is attached
above the ring and on the first carbon. Alpha glucose and beta glucose are isomers and contain
the same number of atoms.
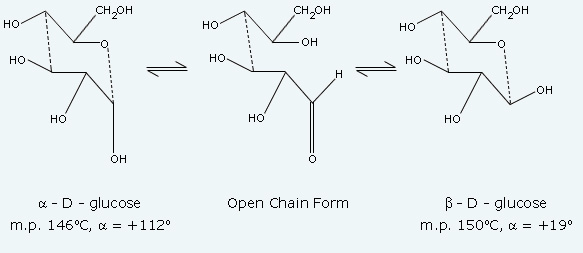
When a D‐(+)‐glucose is dissolved in water, the specific rotation of the sample decreases from a value of +112° to a value of +53°. The specific rotation of 112° corresponds to a D‐(+)‐glucose. The specific rotation of +53° corresponds to an equilibrium mixture of α ‐ D‐(+)‐glucose and β ‐D‐(+)‐glucose. A change in the optical rotation of freshly prepared solutions of sugars is called mutarotation. The forms of glucose involved in mutarotation are the open chain form and two cyclic forms, which are in equilibrium.
The 2 cyclic forms are diastereomers, which have different physical properties(different m.p. and specific rotations). They are referred to as anomers since they differ only in the configuration at the hemiacetal carbon. If either cyclic compound is dissolved in water, the specific rotation changes to +53°. The specific rotation decreases from +112° to +53° when α-D-(+)-glucose is dissolved in water and increases from +19° to +53° when β-D-(+)-glucose is dissolved in water. The mutarotation of glucose is acid-catalyzed and proceeds rapidly at a pH of 7.