Halogen–oxygen bonds are highly covalent due to the similarities in electronegativity of halogens and oxygen. Fluorine is more electronegative than oxygen; therefore compounds of fluorine with oxygen are taken as fluorides of oxygen instead of oxides of fluorine.
| Oxidation State | Fluorine | Chlorine | Bromine | Iodine |
|---|---|---|---|---|
| + 1 | OF2 | Cl2O | Br2O | – |
| + 2 | F2O2 | – | ||
| + 3 | – | Cl2O3 | ||
| + 4 | – | ClO2 | BrO2 | I2O4 |
| + 5 | – | – | – | I2O5 |
| + 6 | – | Cl2O6 | – | – |
| + 7 | – | Cl2O7 | – | – |
Only the structures of OF2, Cl2O, Br2O, Cl2O7 and I2O5 are definitely known. Structures of the monoxides can be illustrated on the basis of VSEPR theory. These oxides have tetrahedral structure having two lone pairs on oxygen. Therefore, the molecule is 'V' shaped or angular in shape.
This is due to electrons in case of OF2 are closer to fluorine because of high electronegativity of F as compared to Cl or Br. The bonded electron pairs in Cl2O and Br2O are closer to oxygen making the repulsion between them more and thus reducing the lone pair-lone pair repulsion on oxygen to certain extent.
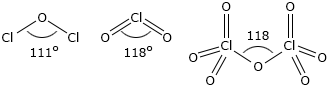
Cl2O: When heated or subject to a spark, Cl2O explodes to Cl2and O2. Dichlorine monoxide reacts with water to form an orange-yellow solution of hypochlorous acid.
ClO2: Chlorine dioxide is a yellowish gas at room temperature .The structure of ClO2 is equivalent to SO2 with one extra electron, resulting in a paramagnetic unpaired electron species.
Cl2O7: Dichlorine heptoxide is a relatively stable oil. Dehydration of perchloric acid with P4O10 produces Cl2O7.Hence the heptoxide is the anhydride of perchloric acid.