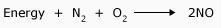Chemical reactions are truly the heart of chemistry, and their applications abound. For instance, if a magician ignites a sheet of nitrocellulose, also known as flash paper. In a moment, it will appear to have vanished. You know from the law of mass conservation, however, that materials don't simply vanish. Rather, they are transformed to new materials. Sometimes we can't see the new materials, but that doesn't mean they don't exist. One of the reactions that occur as flash paper burns is
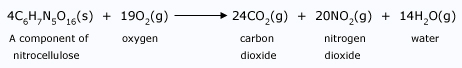
The equation shows 24 carbon, 28 hydrogen, 20 nitrogen, and 102 oxygen atoms before and after the reaction. The difference is in how these atoms are grouped together. The products formed in this case are all gaseous materials that quickly mix into the atmosphere, escaping our notice. To make the flash paper, the magician have to mix the starting materials cellulose and nitric acid. He could determine the proper proportions by knowing the formula masses of these two substances. And although the flash paper may be bathed in an atmosphere of oxygen, it will not react with the oxygen until an initial amount of energy (from the spark of the magician's lighter) is provided to overcome the energy barrier.
We know the burning of flash paper is exothermic because the amount of energy released as product bonds form is greater than the amount absorbed as reactant bonds break. The energy released is in the form of light and faster–moving molecules, which is why the air where the flash paper once was is now appreciably warmer. No true magic is involved, but it is enchanting all the same.
Heat of formation for NO

The amount of energy absorbed as the chemical bonds in the reactants break is

The amount of energy released upon the formation of bonds in the products is
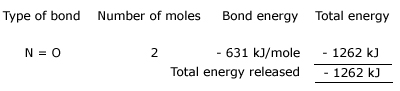
As before, the net energy of the reaction is found by adding the two quantities:
Net energy = energy absorbed + energy released
= +1444 kJ +(– 1262 kJ) = +182 kJ.
The positive sign indicates that there is a net absorption of energy, meaning the reaction is endothermic.
For any endothermic reaction, energy can be considered a reactant and is thus sometimes included before the arrow of the chemical equation: