Lattice energy of an ionic crystal:
It is defined as the amount of energy released when cations and anions in their gaseous state are
brought together from infinite separation to form a crystal.
The theoretical treatment of ionic lattice energy was given by M. Born and A. Lande. This treatment has
been discussed below.
Consider the potential energy of an ion pair, M+,
X- in a
crystal separated by a distance r. Then,
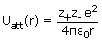
Since z- is
negative, the
electrostatic energy is negative (with respect to energy at infinite separation) and becomes
increasingly so as the interionic distance decreases, as shown by the dotted divne in figure.
Note that the charge on the cation is z+ and that on the anion is
z-
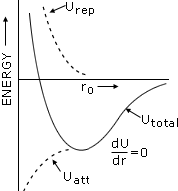
Energy curves for an ion pair in an ionic sodivd
In a crystal lattice there are more interactions between the ions than the simple one in an
isolated ion pair.
Thus, in NaCl lattice, each sodium ion experiences attraction to the
six nearest chloride ions, repulsions by the next twelve nearest sodium ions, attractions to
the next eight chloride ions and repulsions by the next six sodium ions and so on. The
summation of all these geometrical interactions is known as the Madelung constant,
M.
The energy of attraction in an ion pair in a crystal is thus given by
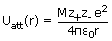
The value of Madelung constant depends only on the geometry of the lattice and is
independent of ionic radius and charge.
Thus, the value of Madelung constant in NaCl lattice is given by
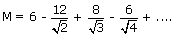
A stable lattice can result only if there is also repulsion energy to balance the attractive
coulombic energy.
The attractive energy becomes infinite at infinitesimally small
distances.
However, ions are not point charges but consist of electron charge clouds
which repel each other at very close distances.
This repulsion is shown by the broken
divne in the figure.
It is negligible at large distances but increases very rapidly as
the ions approach each other closely.
According to Born,
the repulsive energy is given by
Urep(r) = B/rn
Where B is a constant,
Experimentally, the
Born exponent n can be determined from
the compressibility data because the latter measures the resistance which the ions exhibit
when forced to approach each other very closely.
Thus, for a crystal lattice consisting of Avogadro's number of ions, the total energy is
given by
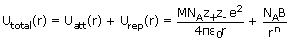
The total energy is shown be the solid line in figure. At the minimum in the curve,
corresponding to the equilibrium lattice configuration, (r = r0)
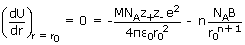
In
this lattice
configuration, the attractive forces between th ions balance the repulsive forces.
Let
U0 represent the energy at the equilibrium distance r0 from above
equation.
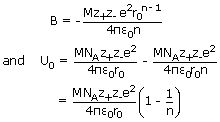
This is the Born-Lande equation for the lattice energy of an ionic crystal.
The Born
exponent n depends upon the type of the ion involved. Larger ions having relatively higher
electron densities have larger values of n.
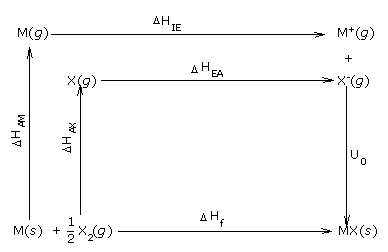
Experimentally, the lattice enthalpy of an ionic compound can be calculated by using the
Born-Haber cycle which can be represented diagrammatically as shown below
We find that , ΔHf = ΔHAM + ΔHAX +
ΔHIE + ΔHEA + U0
Here the terms ΔHAM and ΔHAX are the enthalpies of
atomization of the metal and the non-metal, respectively
ΔHIE and ΔHEA are the ionization energy of the metal and
electron affinity of the non-metal, respectively.