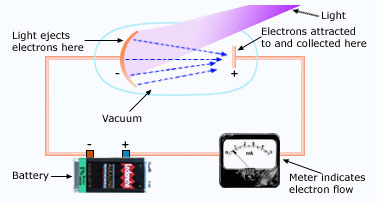
 An apparatus used to study the photoelectric effect.
An apparatus used to study the photoelectric effect.
If you direct a beam of light of short wavelength onto a clean metal surface, the light will cause electrons to leave that surface (light will eject the electrons from the surface). This photoelectric effect is used in many devices, including TV cameras, electric eyes, in the photographer's light meter, night vision viewers and in picking up sound from the sound track of motion pictures. An arrangement for observing the photoelectric effect is shown below:
- The time lag between turning on the light and the ejection of the first electrons was not affected by the brightness or frequency of the light.
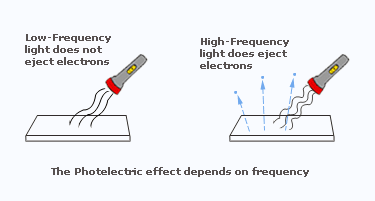
- The effect was easy to observe with violet or ultraviolet light but not with red light.
- The rate at which electrons were ejected was proportional to the brightness of the light.
- The maximum kinetic energy of the ejected electrons was not affected by the brightness(intensity) of the light. However, there were indications that the electron's energy did depend on the frequency of the light.


These observations are a puzzle for Classical physics, which states that light is an electromagnetic wave.
Einstein summed up the results and solved the puzzle using Planck's quantum theory of radiation (light and radiation are words that are interchanged frequently). Planck had assumed that the emission of light in quanta was due to restrictions on the vibrating atoms that produced the light. That is, he assumed that energy in matter is quantized, but that radiant (or light) energy is continuous. Einstein, on the other hand, attributed quantum properties to light itself and viewed it as a hail of particles. To emphasize this particle aspect, we speak of photons whenever we think of the particle nature of light. Now the energy that can be transferred from the incident light to an electron in the metal surface is that of a single photon. One photon is completely absorbed by each electron ejected from the metal. The absorption is an all–or–nothing process and is immediate, so there is no delay as "wave energies" build up.
Absorbing a photon of light may excite an atom. From the relationship E = hf, we see that high–frequency light, such as ultraviolet, which lies beyond the visible spectrum, delivers more energy per photon than lower–frequency light. A light wave has a broad front, and its energy is spread out along this front. For the light wave to eject a single electron from a metal surface, all its energy would somehow have to be concentrated on that one electron. But this is improbable. Therefore, instead of thinking of light encountering a surface as a continuous train of waves, the photoelectric effect suggests that we conceive of light encountering a surface or any detector as a succession of corpuscles, or photons. The number of photons in a light beam controls the brightness of the whole beam, whereas the frequency of the light controls the energy of each individual photon. The photoelectric effect proves conclusively that light has particle properties. We cannot conceive of the photoelectric effect on the basis of waves.