To understand the process of evaporation, do the following experiments :
1. Two vessels whose mouths have different areas. Fill the vessels with water and keep them in open air. Note their levels with a marker pen. After a few hours, note their levels again. You will observe that the level in vessel with a wider mouth and larger exposed water area has gone down more than what has happened in the other vessel. This shows that the rate of evaporation depends on the exposed area of the liquid.
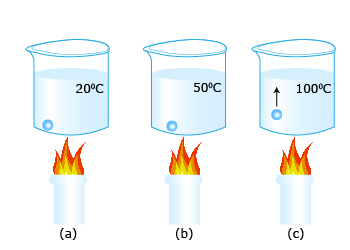
2.Take equal amounts of water at different temperatures. Put them in exactly similar vessels with similar mouths. Notice the water levels after a short passage of time. You will observe that the water at higher temperature evaporates faster. Thus the rate of evaporation depends on the temperature of the liquid.
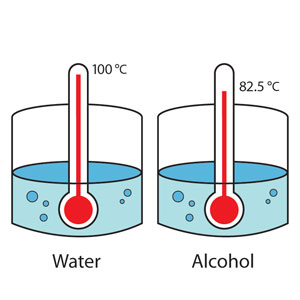
3.Take equal amounts of water and alcohol at the same temperature. Put them in identical vessels and observe the levels over a short period of time. You will observe that despite identical conditions, alcohol evaporates faster than water. This shows that the rate of evaporation varies with the nature of the liquid.
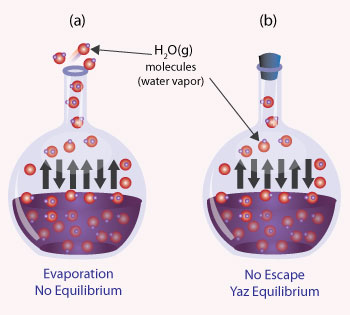
4. Take equal amounts of water in identical vessels. Mark the level of water with a marker pen. Cover one of the vessels with a belly jar. Note the level of water with respect to time. You will see that the uncovered water level goes down faster. This shows that the rate of evaporation depends on whether it takes place in the open or in a closed space.