Know why ?
Q.
Why Ga is liquid at room temperature ?
Sol.
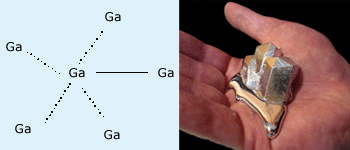
The element with least melting point in 13th group is Ga with
melting point of 30°C
In 13th group, Boron has high melting point, due to its compact icosahedral structure. Ga has low melting point because it exists as dimer in its crystalline structure. Gallium has wide range of liquid state from 30°C – 2000°C. So it can be used in high temperature thermometers. From Ga to Tl melting point increases due to poor shielding f and d electrons which enhances the metal to metal cohesive forces.
Melting point order: B > Al > Ga < In < Tl