What is acid rain?
As rainwater falls, it absorbs atmospheric carbon dioxide. Once in the rainwater, the carbon dioxide reacts with water to form an acid known as carbonic acid, H2CO3, which makes rainwater naturally acidic.
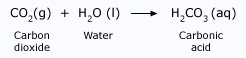
Carbonic acid, as its name implies, behaves as an acid and lowers the pH of water. The CO2 in the atmosphere brings the pH of rainwater to about 5.6 noticeably below the neutral pH value of 7. Because of local fluctuations, the normal pH of rainwater varies between 5 and 7. This natural acidity of rainwater may accelerate the erosion of land and, under the right circumstances, can lead to the formation of underground caves. By convention, acid rain is a term used for rain having a pH lower than 5.
Acid rain is a major environmental problem common to all industrialized countries. This is caused by heavy pollution. Large amount of coal is burnt in industries. Coal contains sulphur as impurity. Burning of coal also leads to formation of oxides of sulphur like SO2 and SO3. Emission from cars contains oxides of Nitrogen like NO2. These oxides dissolve in the water vapor in the atmosphere. This makes the rainwater acidic. When such acid rain falls, the crops get affected, the yield is reduced Sulphur dioxide is readily converted to sulfur trioxide, which reacts with water to form sulphuric acid.
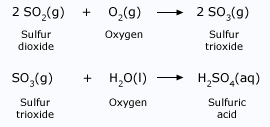
Acid rain water gets mixed with water in lakes and cattle bathing in such lakes develop skin lesions like burns. It also affects buildings and statues.
Farmers neutralize the effects of acid rain in the soil by adding slaked lime or calcium hydroxide to the soil.
People suffering from acidity of stomach take antacid medicines. Explain?
Our stomach wall secretes mild HCl for breaking down food molecules. But some people suffer from what they describe as "ACIDITY". They complain of severe nausea and vomiting sensation. Or even burning sensation in the stomach and esophagus. This happens mainly when a person is over eating or eating spicy, oily food. The stomach lining starts excreting more acid than required. Such people are advised to take antacid tablets - antacids contain hydroxides, such as magnesium hydroxide or aluminium hydroxide. These hydroxides neutralize the acid in the stomach.
People with "motion sickness"tend to travel empty stomach. The motion of a vehicle causes the stomach acid to be stirred up. Actually they need to be advised never to travel empty stomach because it is the acid, which is causing this trouble.
What is aqua regia?
Aqua regia is a mixture of Nitric acid and Hydrochloric acid in a definite proportion (HNO3 : HCl :: 1 : 3).This becomes stronger than sulphuric acid. Royal metals like gold and silver do not react with any of the ordinary acids (including sulphuric acid). These royal metals react with aqua regia.
Why do our stomachs feel strange when we get hungry?
When food enters stomach HCl secreted in the stomach digests it. When we get used to eating food at a specific time our body cells secrete HCl even before we eat anything. Actually it is this secretion of acid that tells us "You are hungry".
Why are fruit juices and salts stored in glass or plastic bottles or paper carton?
Fruit juices such as orange juice, lemon juice are acidic. In fact a lot of fruit juices, a sour taste is deliberately added. This again is citric acid. Acids react with metal vessels. On the other hand acid does not react with glass or plastic. Hence fruit juices are stored in glass or plastic bottles. If you look inside a paper carton that have fruit juices, there will be a layer of thin plastic sheet inside. The plastic sheet will not react with acids.Similarly, salt is also never sold or stored in metal containers. Salt, in presence of moisture will convert itself into a base. It is likely that the base will react with metal and get corroded.