Preparation of Aldehydes
From acyl chloride (acid chloride)

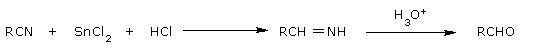
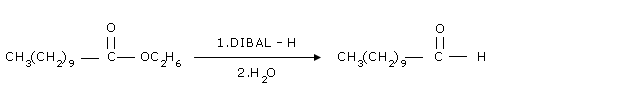
Use of chromic oxide (CrO3):
- Toluene or substituted toluene is converted to benzylidene diacetate on treating
with chromicoxide in acetic anhydride.
The benzylidene diacetate can be hydrolyzed to corresponding benzaldehyde with
aqueous acid.

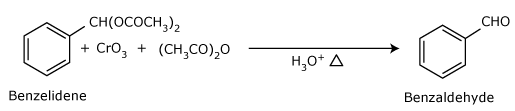
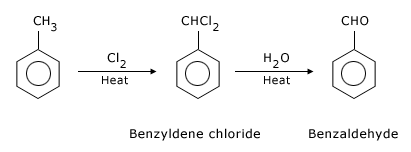
- By side chain chlorination followed by hydrolysis
Side chain chlorination of toluene gives benzal chloride, which on hydrolysis gives
benzaldehyde.
This is a commercial method of manufacture of benzaldehyde.
F Hydrolysis of side chain chlorination of toluene.
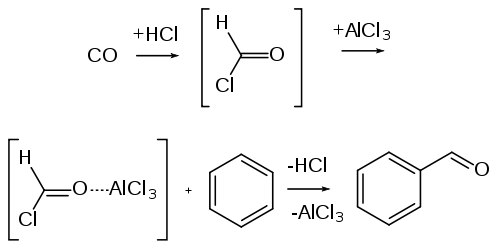
- By Gatterman - Koch reaction
When benzene or its derivative is treated with carbon monoxide and hydrogen chloride
in the presence of anhydrous aluminum
chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.
This reaction is known as Gatterman-Koch reaction.
Gatterman-Koch reaction.
Preparation of Ketones
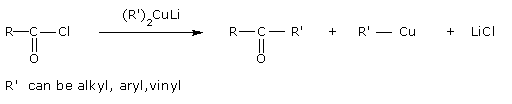
- From acyl chlorides
Treatment of acyl chlorides with dialkylcadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.
- From nitriles
Treating a nitrile with Grignard reagent followed by hydrolysis yields a ketone.
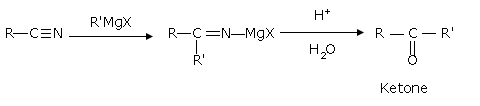
Hydrolyzedphysical properties
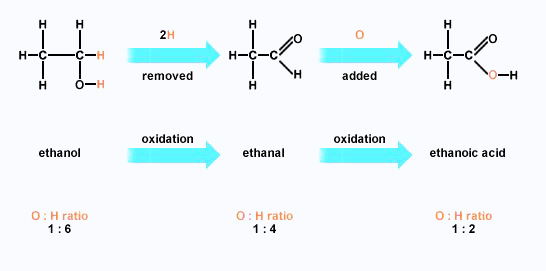
Polarity Butanal is more polar than ethoxyethane.
Physical state Aldehydes and Ketones are normally liquids at room temperature.

Odor Aldehydes and ketones are identified by their characteristic odor and flavor. The lower aldehydes have sharp pungent odors. As the size of the molecule increases, the odor becomes less pungent and more fragrant. In fact, many naturally occurring aldehydes and ketones are used in the blending of perfumes and flavoring agents.
Boiling points Methanal is a gas (boiling point - 21°C), and ethanal has a boiling point of + 21°C. That means that ethanal boils at close to room temperature.
The other aldehydes and the ketones are liquids, with boiling points rising as the molecules get bigger.
Van der Waals dispersion forces These attractions get stronger as the molecules get longer and have more electrons. That increases the sizes of the temporary dipoles that are set up. This is why the boiling points increase as the number of carbon atoms in the chains increases irrespective of whether you are talking about aldehydes or ketones.
Vander Waals dipole-dipole attractions Both aldehydes and ketones are polar molecules because of the presence of the carbon-oxygen double bond. As well as the dispersion forces, there will also be attractions between the permanent dipoles on nearby molecules.
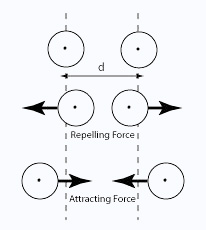
That means that the boiling points will be higher than those of similarly sized hydrocarbons which only have dispersion forces. It is interesting to compare three similarly sized molecules. They have similar lengths, and similar (although not identical) numbers of electrons.
| Molecule | Type | Boiling point (°C) |
|---|---|---|
| CH3CH2CH3 | alkane | - 42 |
| CH3CHO | aldehyde | + 21 |
| CH3CH2OH | alcohol | + 78 |
Notice that the aldehyde (with dipole-dipole attractions as well as dispersion forces) has a boiling point higher than the similarly sized alkane which only has dispersion forces. However, the aldehyde's boiling point isn't as high as the alcohol's. In the alcohol, there is hydrogen bonding as well as the other two kinds of intermolecular attraction. Although the aldehydes and ketones are highly polar molecules, they don't have any hydrogen atoms attached directly to the oxygen, and so they can't hydrogen bond with each other.
N-Butane 273 b.p.(K) ; 58 Molecular Mass Methoxyethane 281b.p.(K) ; 60 Molecular Mass Propanal 322 b.p.(K) ; 58 Molecular Mass Acetone 329 b.p.(K) ; 58 Molecular Mass Propan-1-ol 370 b.p.(K) ; 60 Molecular Mass Hence increasing order of boiling points of the given compounds is as follows: CH3CH2CH2CH2CH3 < H5C2 - O - C2H5 < CH3CH2CH2CHO < CH3CH2CH2CH2OH
The molecular masses of these compounds are in the range of 72 to74.
Solubility in water The small aldehydes and ketones are freely soluble in water but solubility falls with chain length.
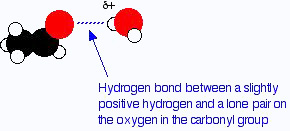
Example:methanal, ethanal and propanone the common small aldehydes and ketones are miscible with water in all proportions. The reason for the solubility is that although aldehydes and ketones can't hydrogen bond with themselves, they can hydrogen bond with water molecules.
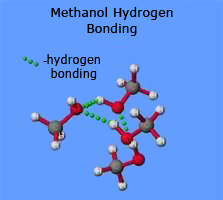
One of the slightly positive hydrogen atoms in a water molecule can be sufficiently attracted to one of the lone pairs on the oxygen atom of an aldehyde or ketone for a hydrogen bond to be formed.
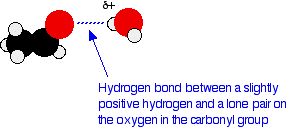
There will also, of course, be dispersion forces and dipole-dipole attractions between the aldehyde or ketone and the water molecules. Forming these attractions releases energy which helps to supply the energy needed to separate the water molecules and aldehyde or ketone molecules from each other before they can mix together. As chain lengths increase, the hydrocarbon "tails" of the molecules (all the hydrocarbon bits apart from the carbonyl group) start to get in the way. By forcing themselves between water molecules, they break the relatively strong hydrogen bonds between water molecules without replacing them by anything as good. This makes the process energetically less profitable, and so solubility decreases.
- Acetone:Green clover leafs release acetone into the atmosphere which is stable in the lower atmosphere ,but undergoes photolysis, contributing to the oxidative balance of the Troposphere-stratosphere boundary.
 Green clover leafs
Green clover leafs
 Field measurement of the
Field measurement of the release of the compound.
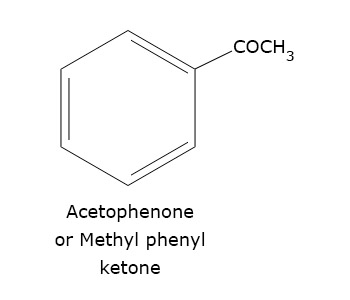
- Acetophenone
Acetophenone is naturally found in apple, cheese, apricot, banana and cauliflower.
Acetophenone is used in the production of synthetic musk for perfume industry.
 Apricot tree
Apricot tree
 Synthetic musk
Synthetic musk
- Benzophenone Benzophenone-3 occurs naturally in some flowering plants and protects the plant from UV rays. Because of the ability to absorb and scatter the UV rays benzophenone-3 is used in some lotions and cosmetics as a sunscreen. These names shown above are still considered to be acceptable IUPAC names.


- Ethyl isopropyl ketone
- Diethyl ketone The first three of the names shown above are still considered to be acceptable IUPAC names.
Aldehydes

The common name for an aldehyde is derived from the common name of the corresponding carboxylic acid by dropping the word acid and changing the suffix from - ic or - oic to - aldehyde.
- Formaldehyde Formaldehyde is used in preserving the specimen in labs.
- Acetaldehyde At low concentrations acetaldehyde is associated with green apples, bruised apples, emulsion paint, wine(white wine).


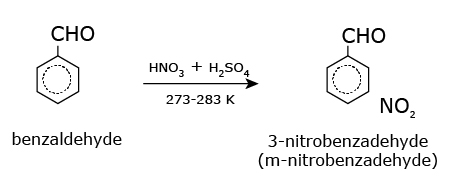
- Benzaldehyde
Benzaldehyde is obtained from almond extracts and herbal extracts and is used as an intermediate in organic synthesis and in organic perfumes as flavor and fragrance.
 At the same time, the names reflect the Latin or Greek term for the original source of the acid or aldehyde.The location of the substituting the carbon chain is indicated by Greek letters α, β, .., γ, etc. The α - carbon being the one directly linked to the aldehyde group β carbon the next, and so on.
At the same time, the names reflect the Latin or Greek term for the original source of the acid or aldehyde.The location of the substituting the carbon chain is indicated by Greek letters α, β, .., γ, etc. The α - carbon being the one directly linked to the aldehyde group β carbon the next, and so on.

Ketones
The common names of ketones are derived by naming two alkyl or aryl groups bonded to the carbonyl group. The locations of substituents are indicated by Greek letters, α α', β , β' and so on beginning with the carbon atoms next to the carbonyl group, indicated as αα'. Some ketones have historical common names, the simplest dimethyl ketone is called acetone. Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
IUPAC names
The IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending e with al and one respectively. In case of aldehydes the longest carbon chain is numbered starting from the carbon of the aldehyde group while in case of ketones the numbering begins from the end nearer to the carbonyl group.


The substituents are prefixed in alphabetical order along with numerals indicating their positions in the carbon chain. The same applies to cyclic ketones, where the carbonyl carbon is numbered one.


When the aldehyde group is attached to a ring, the suffix carbaldehyde is added after the full name of the cycloalkane. The numbering of the ring carbon atoms start from the carbon atom attached to the aldehyde group. The name of the simplest aromatic aldehyde carrying the aldehyde group on a benzene ring is benzenecarbaldehyde. However, the common name benzaldehyde is also accepted by IUPAC. Other aromatic aldehydes are hence named as substituted benzaldehydes.
Common and IUPAC Names of Some Aldehydes and Ketones
Aldehydes
- HCHO ----- Formaldehyde
- CH3CHO ----- Acetaldehyde Ethanal
- (CH3)2CHCHO ----- Isobutyraldehyde 2-Methylpropanal
- CH3CH2CH2CH2CHO ------ Valeraldehyde Pentanal
- CH2 = CHCHO Acrolein Prop-2-enal
Ketones
- CH3COCH2CH2CH3 ------ Methyl n-propyl ketone
- Pentan-2-one
- (CH3)2CHCOCH(CH3)2 -----Diisopropyl ketone 2,4-Dimethylpentan-3-one
STRUCTURE OF THE CARBONYL GROUP
The carbonyl carbon atom is sp2-hybridized and forms three sigma (s) bonds. The fourth valence electron of carbon remains in its p-orbital
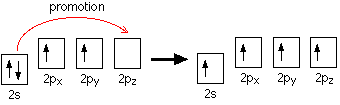
Hybridization of the 2s orbital and two of the 2p orbitals means that the carbon atom now looks like the diagram
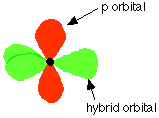
Three sp2 hybrid orbitals are formed and these arrange themselves as far apart in space as they can at 120° to each other. The remaining p orbital is at right angles to them.
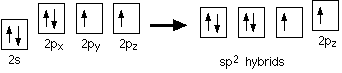
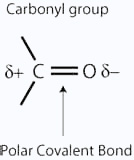
This sideways overlap produces a pi bond. So just like C = C, C = O is made up of a sigma bond and a pi bond. The carbon-oxygen double bond is polarized due to higher electronegativity of oxygen relative to carbon. Hence, the carbonylcarbon is an electrophilic center (Lewis acid), and carbonyloxygen, a nucleophilic (Lewis base) center. Carbonyl compounds have substantial dipole moments and are polar than ethers. The high polarity of the carbonyl group is explained on the basis of resonance involving a neutral and a dipolar.
The ketone carbon is often described as "sp2 hybridized", terminology that describes both their electronic and molecular structure. Ketones are trigonal planar about the ketonic carbon, with C - C - O and C - C - C bond angles of approximately 120°.
Ketones are not usually hydrogen-bond donors and cannot hydrogen-bond to itself. Because of their inability to serve both as hydrogen-bond donors and acceptors, ketones tend not to "self-associate" and are more volatile than alcohols and carboxylic acids of comparable molecular weights.

As the ketones are volatile, they can be smelled readily. The rosy aroma of a rose is because of the Ketones. The higher the level of ketones in a rose, the higher is the rosy aroma of a rose.
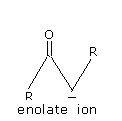
These factors relate to pervasiveness of ketones in perfumery and as solvents. A ketone that has an α - hydrogen participates in a so called keto-enol tautomerism. The reaction with a strong base gives the corresponding enolate, often by deprotonation of the enol.

- keto form
- eno form
Ketones that have at least one α - hydrogen, undergo keto-enol tautomerization; the tautomer is an enol. Tautomerization is catalyzed by both acids and bases. Usually, the keto form is more stable than the enol. This equilibrium allows ketones to be prepared via the hydration of alkynes.
Classes of ketones
Ketones are classified on the basis of their substituents as
- Symmetrical and Unsymmetrical ketones
- Diketones
- Unsaturated ketones
- cyclic ketones
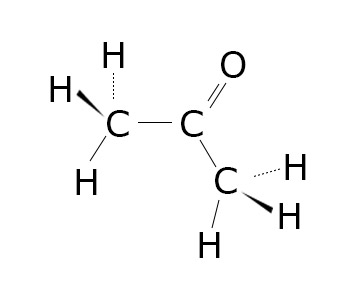
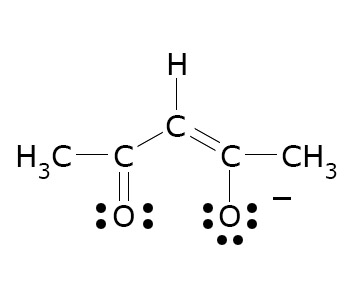
Diketones
Many kinds of diketones are known, some with unusual properties. Its enolate is a common ligand in coordination chemistry.
- Diacetyl (CH3C(O)C(O)CH3), once used as butter-flavoring in popcorn.
- Acetylacetone (pentane-2,4-dione) is virtually a misnomer (inappropriate name)
because
this species exists mainly as the monoenol CH3C(O)CH =
C(OH)CH3.
Unsaturated ketones
Ketones containing alkene and alkyne units are often called unsaturated ketones.
EXAMPLE:
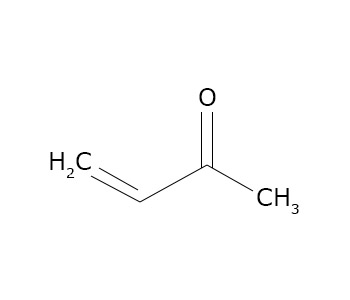
Methyl vinyl ketone contains an alkene and an alkyne group attached to the carbonyl carbon, hence called as unsaturated Ketone.
Cyclic ketones
Many ketones are cyclic. The simplest class have the formula (CH2)nCO, where n varies from 3 for cyclopropanone to the teens. Larger derivatives exist.
EXAMPLE:
- Cyclohexanone, a symmetrical cyclic ketone, is an important intermediate in the
production of nylon.
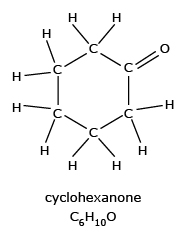
 Cyclohexanone is an important commercial product, used to obtain caprolactum for nylon-6 production which are used as threads in bristles for tooth brushes, surgical sutures and strings for acoustic and musical instruments.
Cyclohexanone is an important commercial product, used to obtain caprolactum for nylon-6 production which are used as threads in bristles for tooth brushes, surgical sutures and strings for acoustic and musical instruments.
- Isophorone, derived from acetone, is an unsaturated, unsymmetrical ketone that is
the precursor to other polymers.
Isophorone
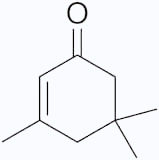 Isophorone is used as an ingredient in wood preservative and floor sealants.
Isophorone is used as an ingredient in wood preservative and floor sealants.
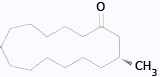
- Muscone, 3-methylpentadecanone, is an animal pheromone.
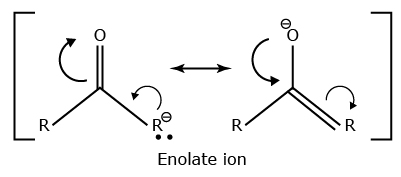
The relative acidity of the α- hydrogen is important in the enolization reactions of ketones and other carbonyl compounds. The acidity of the α - hydrogen also allows ketones and other carbonyl compounds to undergo nucleophilic r eactions at that position, with either stoichiometric and catalytic base.
The isomers which differ in the length of carbon chain are called chain isomers. Aldehydes which contain four or more carbon atoms, and the ketones which contain five or more carbon atoms show chain isomerism.
- Butanal (n-butyral-dehyde) and methylpropan-2-al (iso-butyraldehyde) are chain
isomers.
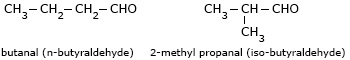
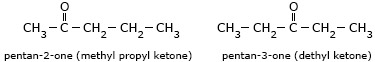
- Pentan-2-one (methyl propyl ketone) and 3-methylbutan-2-one (methyl isopropyl
ketone)
are chain isomers.
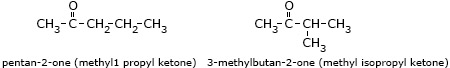
Aliphatic aldehydes do not show position isomerism, because the - CHO group is always present at the end of the chain.
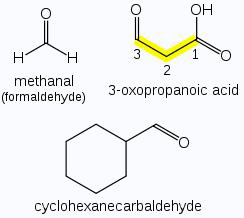
Aliphatic aldehydes and ketones having the same number of carbon atoms can be represented by the same molecular formula. Therefore, such an aldehyde and ketone are functional isomers of each other.
- All Aldehydes (RCHO) and ketones (RCOR) contain a C = O bond.
- The C = 0 bond is polar and the electrons are attracted to the oxygen atom The oxygen atom reacts with electrophiles and the carbon atom reacts with nucleophiles.
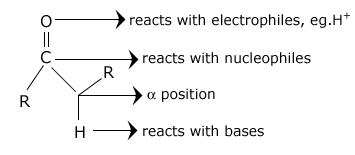
The position of carbon atom next to C = O is called the α - position Deprotonation of an aldehyde or ketone at the α - position forms an enolate ion.
- Tollens' test:
On warming an aldehyde with freshly prepared ammoniacal silver nitrate solution (Tollens' reagent), a bright silver mirror is produced due to the formation of silver metal.Tollen's reagent contain ammonical silver nitrate along with ethanol.
 The aldehydes are oxidized to corresponding carboxylate anion. The reaction occurs in alkaline medium.
The aldehydes are oxidized to corresponding carboxylate anion. The reaction occurs in alkaline medium.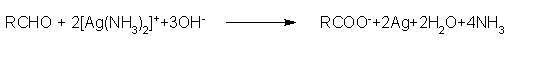
- Fehling's test:
Fehling reagent comprises of two solutions, Fehling solution A and Fehling solution B. Fehling solution A is aqueous copper sulphate and Fehling solution B is alkaline sodium potassium tartarate (Rochelle salt).Fehling solution A and Fehling solution B. These two solutions are mixed in equal amounts before test. On heating an aldehyde with Fehling's reagent, a reddish brown precipitate is obtained.
 Aldehydes are oxidized to corresponding carboxylate anion. Aromatic aldehydes do not respond to this test.
Aldehydes are oxidized to corresponding carboxylate anion. Aromatic aldehydes do not respond to this test.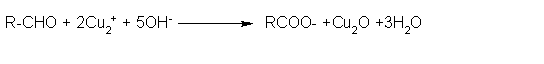
- Benedict's test
Benedict's reagent contains blue copper(II) ions (Cu2+) which are reduced to copper(I) ions (Cu+). These are precipitated as red copper(I) oxide which is insoluble in water.Blue colored Benedict's reagent changes colors on reacting with aldehydes and ketones. Benedict's reagent is prepared from copper sulfate penta hydrate, sodium citrate and anhydrous sodium carbonate. Benedict's reagent is used as a test for the presence of reducing sugars. This includes all monosaccharides and the disaccharides, lactose and maltose. Even more generally, Benedict's test will detect the presence of aldehydes (except aromatic ones), and alpha–hydroxy–ketones, including those that occur in certain ketoses.
 Thus, although the ketose fructose is not strictly a reducing sugar, it is an alpha–hydroxy–ketone, and gives a positive test because it is converted to the aldoses glucose and mannose by the base in the reagent.
Thus, although the ketose fructose is not strictly a reducing sugar, it is an alpha–hydroxy–ketone, and gives a positive test because it is converted to the aldoses glucose and mannose by the base in the reagent.
- Are the carbohydrates having hemiacetal structures in equilibrium with a free aldehyde.
- Reducing sugars give positive results for Tollen's or benedicts test.

- Are the carbohydrates that are in acetal form and do not have free aldehyde.
- Non Reducing sugars give negative results for Tollen's or benedicts test.

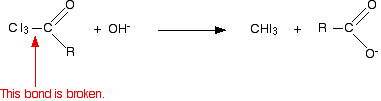
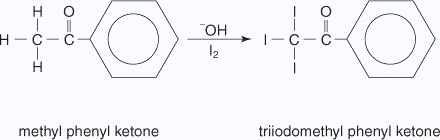
Iodoform reaction with sodium hypoiodite is also used for detection of CH3CO group or CH3CH(OH) group which produces CH3CO group on oxidation.
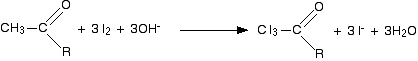
In the second stage, the bond between the C I3 and the rest of the molecule is broken to produce triiodomethane (iodoform) and the salt of an acid.
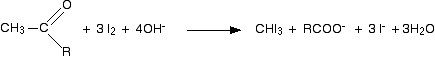
Carbonyl compounds will form polymers by nucleophilic addition across the C = O bond. Ketones do not form polymers easily as they are not particularly reactive, but aldehydes readily form a variety of polymers.
In some cases the polymers are made by simple addition of one monomer to the other - polymethanal, a strong plastic, is one example of this. Ketones do not polymerize, while aldehydes undergo polymerization.


- Nucleophillic addition reactions,
- Nucleophillic addition - elimination reactions,
- α - substituted reactions,and
- carbonyl - reactions.
The most common reactions are nucleophilic addition reactions, which lead to the formation of alcohols, alkenes, diols, cyanohydrins (RCH(OH)CN), and imines( R2CNR).
| Reaction name | Substrate | Comment |
|---|---|---|
| Ozonolysis | alkene | ozonolysis of non-fully-substituted alkenes yield aldehydes upon reductive work-up. |
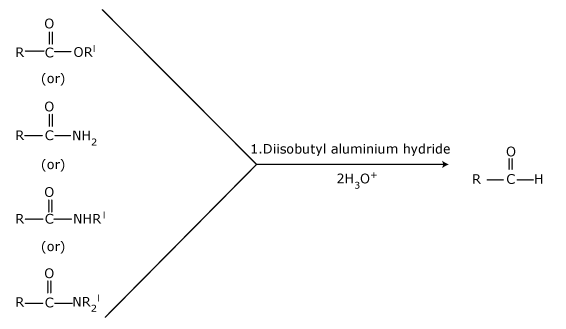
| Organic reduction | ester | Reduction of an ester with diisobutylaluminum hydride (DIBAL-H) or sodium aluminum hydride |
| Rosenmund reaction | acid chloride | or using lithium tri-t-butoxyaluminum hydride (LiAlH(OtBu)3). |
| Wittig reaction | ketone | reagent methoxymethylenetriphenylphosphine in a modified Wittig reaction. |
| Formylation reactions | nucleophilic arenes | various reactions for example the Vilsmeier-Haack reaction |
| Nef reaction | Nitro compound | |
| Zincke reaction | pyridines | Zincke aldehydes form in a variation |
| Stephen aldehyde synthesis | nitriles | reagents tin(II) chloride and hydrochloric acid. |
| Meyers synthesis | oxazine | oxazine hydrolysis |
| McFadyen-Stevens reaction | hydrazide | is a base-catalyzed thermal decomposition of acylsulfonylhydrazides |
- Nucleophillic addition reactions:
The C = O bond of aldehydes and ketones reacts with nucleophiles (such as H-, an organometallic reagent, or CN-)in nucleophillic reactions. Nucleophiles add more rapidly to aldehydes than ketones because of steric and electronic effects. The reactions of nucleophilic groups containing oxygen,sulfur or nitrogen with C = O bond are reversible and are catalyzed by an acid.
Aldehydes and Ketones undergo Nucleophilic addition reactions . A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridized orbitals of carbonyl carbon . The hybridization of carbon changes from sp2 to sp3 in this process,and a tetrahedral alkoxide intermediate is produced. This intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu- and H+ across the carbon oxygen double bond.
- Addition of water
The addition of water to an aldehyde results in the formation of a hydrate.The formation of a hydrate proceeds via a nucleophilic addition mechanism.
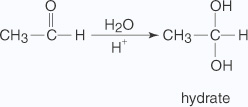
- Water, acting as a nucleophile, is attracted to the partially positive carbon of
the carbonyl group,
generating an oxonium ion.
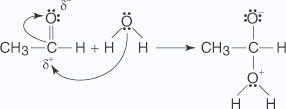
- The oxonium ion liberates a hydrogen ion that is picked up by the oxygen anion in
an acid-base reaction.
Small amounts of acids and bases catalyze this reaction. This occurs because the addition of acid causes a protonation of the oxygen of the carbonyl group, leading to the formation of a full positive charge on the carbonyl carbon, making the carbon a good nucleus.
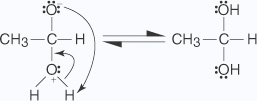 Adding hydroxyl ions changes the nucleophile from water (a weak nucleophile) to a hydroxide ion (a strong nucleophile). Ketones usually do not form stable hydrates.
Adding hydroxyl ions changes the nucleophile from water (a weak nucleophile) to a hydroxide ion (a strong nucleophile). Ketones usually do not form stable hydrates. - Addition of Hydrogen cyanide
Aldehydes and ketones react with hydrogen cyanide (HCN) to yield cyanohydrins. This reaction occurs very slowly with pure HCN. Therefore, it is catalyzed by a base and the generated cyanide ion (CN-) being a stronger nucleophile readily adds to carbonyl compounds to yield corresponding cyanohydrin.Cyanohydrins are useful synthetic intermediates.The addition of hydrogen cyanide to a carbonyl group of an aldehyde or most ketones produces a cyanohydrin. Sterically hindered ketones, however, don't undergo this reaction.The mechanism for the addition of hydrogen cyanide is a straightforward nucleophilic addition across the carbonyl carbony oxygen bond.
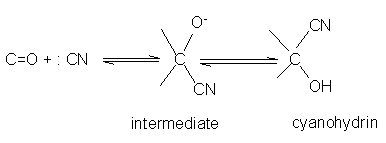
- Addition of sodium hydrogen sulphite(NaHSO3) Sodium
hydrogensulphite adds to aldehydes
and ketones to form the addition products.
The position of the equilibrium lies largely to the right hand side for most aldehydes and to the left for most ketones due to steric reasons.The hydrogensulphite addition compound is water soluble and can be converted back to the original carbonyl compound by treating it with dilute mineral acid or alkali.
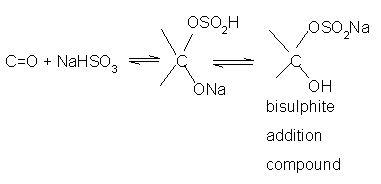 Therefore, these are useful for separation and purification of aldehydes.
Therefore, these are useful for separation and purification of aldehydes. - Addition of alcohol Reactions of aldehydes with alcohols produce either hemiacetals (a functional group consisting of one - OH group and one - OR group bonded to the same carbon) or acetals (a functional group consisting of two -OR groups bonded to the same carbon), depending upon conditions. Mixing the two reactants together produces the hemiacetal. Mixing the two reactants with hydrochloric acid produces an acetal.
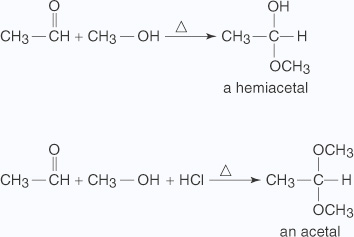
A nucleophilic substitution of an OH group for the double bond of the carbonyl group forms the hemiacetal through the following mechanism:
- An unshared electron pair on the alcohol's oxygen atom attacks the carbonyl
group.

- The loss of a hydrogen ion to the oxygen anion stabilizes the oxonium ion formed in
Step
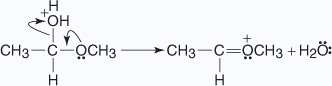
The addition of acid to the hemiacetal creates an acetal through the following mechanism:

- The proton produced by the dissociation of hydrochloric acid protonates the alcohol
molecule in an acid-base reaction.

- An unshared electron pair from the hydroxyl oxygen of the hemiacetal removes a
proton from the protonated alcohol.

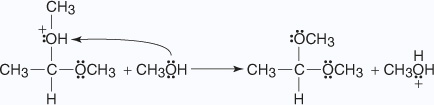
- The oxonium ion is lost from the hemiacetal as a molecule of water.
- A second molecule of alcohol attacks the carbonyl carbon that is forming the
protonated acetal.

- The oxonium ion loses a proton to an alcohol molecule, liberating the acetal.
Acetal formation reactions are reversible under acidic conditions but not under alkaline conditions. This characteristic makes an acetal an ideal protecting group for aldehyde molecules that must undergo further reactions. A protecting group is a group that is introduced into a molecule to prevent the reaction of a sensitive group while a reaction is carried out at some other site in the molecule. The protecting group must have the ability to easily react back to the original group from which it was formed. An example is the protection of an aldehyde group in a molecule so that an ester group can be reduced to an alcohol.
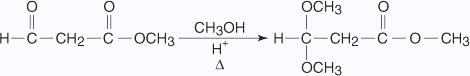
In the previous reaction, the aldehyde group is converted into an acetal group, thus preventing reaction at this site when further reactions are run on the rest of the molecule.
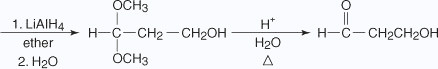
Notice in the previous reaction that the ketone carbonyl group has been reduced to an alcohol by reaction with LiAlH4. The protected aldehyde group has not been reduced. Hydrolysis of the reduction product recreates the original aldehyde group in the final product.
- Addition of ylides (The Wittig reaction)
The reaction of aldehydes or ketones with phosphorus ylides produces alkenes of unambiguous double-bond locations. Phosphorous ylides are prepared by reacting a phosphine with an alkyl halide, followed by treatment with a base. Ylides have positive and negative charges on adjacent atoms.The following illustration shows the preparation of 2-methylbutene by a Wittig reaction.


- Addition of organometallic reagents
Grignard reagents, organolithium compounds, and sodium alkynides react with formaldehyde to produce primary alcohols, all other aldehydes produce secondary alcohols, and ketones to produce tertiary alcohols.

- Nucleophilic addition -elimination reaction:
The nucleophilic addition reactions of aliphatic aldehydes and ketones in which carbonyl oxygen is lost are called as nucleophilic addition elimination reactions.Addition of ammonia and ammonia derivatives to aldehydes and ketones undergo nucleophilic addition elimination reactions.

- An unshared pair of electrons on the nitrogen of the amine is attracted to
the partial-positive carbon of the carbonyl group.
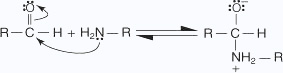
- A proton is transferred from the nitrogen to the oxygen anion.
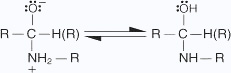
- The hydroxy group is protonated to yield an oxonium ion, which easily liberates a
water molecule
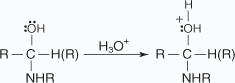
- An unshared pair of electrons on the nitrogen migrate toward the positive oxygen,
causing the loss of a water molecule.
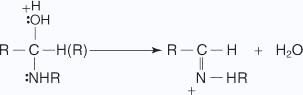
- A proton from the positively charged nitrogen is transferred to water, leading to
the imine's formation.
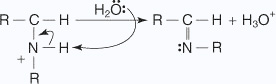
Nucleophiles, such as ammonia and its derivatives H2N - Z add to the carbonyl group of aldehydes and ketones. The reaction is reversible and catalyzed by acid. The equilibrium favors the product formation due to rapid dehydration of the intermediate to form > C = N - Z.
Z = Alkyl, aryl, OH, NH2, C6H5NH, NHCONH2, etc. Imines of aldehydes are relatively stable while those of ketones are unstable. Derivatives of imines that form stable compounds with aldehydes and ketones include:
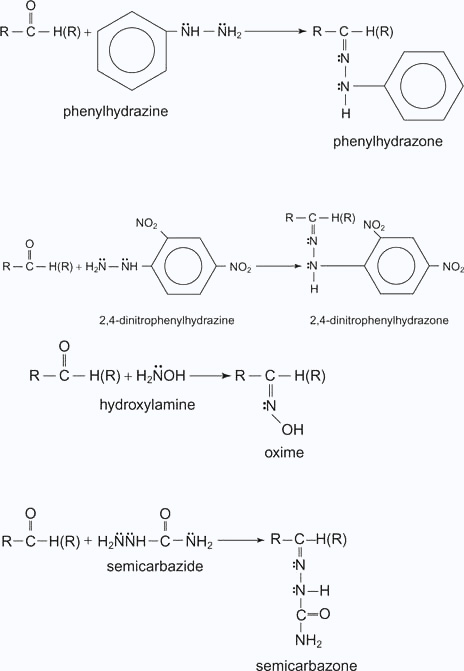
Oximes, 2,4 - dinitrophenylhydrazones, and semicarbazones are often used in qualitative organic chemistry as derivatives for aldehydes and ketones.
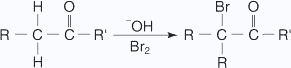
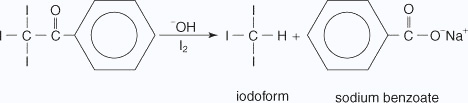
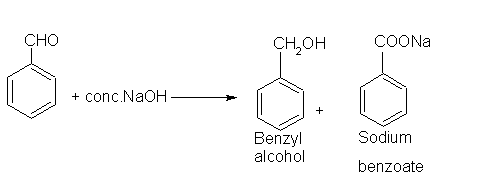
The generation of sodium hypoiodate in solution from the reaction of iodine with sodium hydroxide leads to the formation of iodoform and sodium benzoate, as shown here.
- The base removes an α hydrogen.
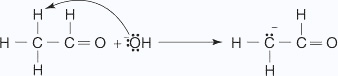
- The carbanion undergoes nucleophilic addition with the carbonyl group of a
second molecule of ethanal,
which leads to formation of the condensation product.
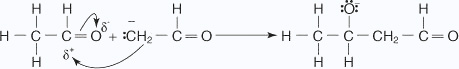
- A reaction with water protonates the alkoxide ion.
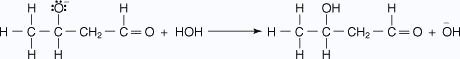
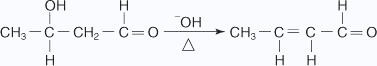
Ketonic aldol condensation
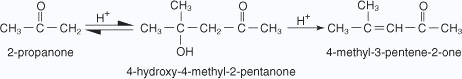
The acid-catalyzed aldol condensation includes two key steps: the conversion of the ketone into its enolic form, and the attack on a protonated carbonyl group by the enol. The mechanism proceeds as follows:
- The oxygen of the carbonyl group is protonated.
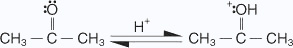
- A water molecule acting as a base removes an acidic a hydrogen, which leads to
an enol.
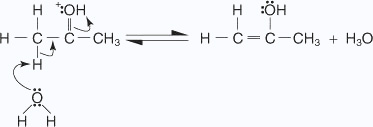
- The enol attacks a protonated carbonyl group of a second ketone molecule.
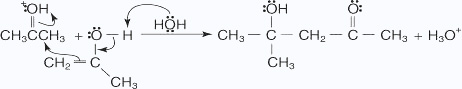
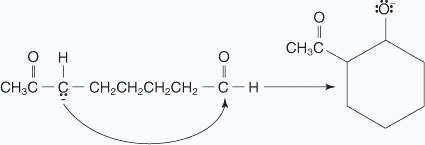
- The hydroxy ion removes a hydrogen ion α to the ketone carbonyl.

- The enolate ion attacks the aldehyde carbonyl, closing the ring.
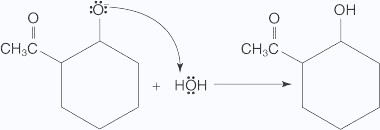
- The alkoxide ion abstracts a proton from water in an acid–base reaction.
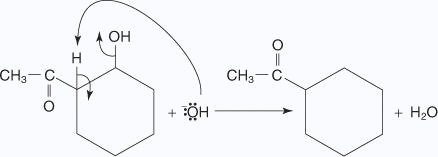
- The base removes a hydrogen ion to form a resonance-stabilized molecule.
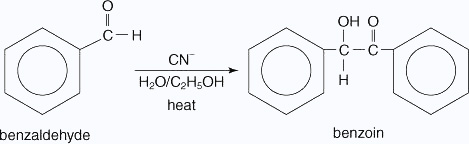
The cyanide ion is the only known catalyst for this condensation, because the cyanide ion has unique properties. For example, cyanide ions are relatively strong nucleophiles, as well as good leaving groups. Likewise, when a cyanide ion bonds to the carbonyl group of the aldehyde, the intermediate formed is stabilized by resonance between the molecule and the cyanide ion. The following mechanism illustrates these points.
- The cyanide ion is attracted to the carbon atom of the carbonyl group.
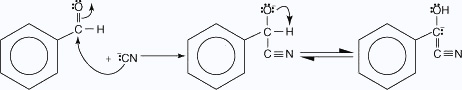
- The carbanion is resonance–stabilized.
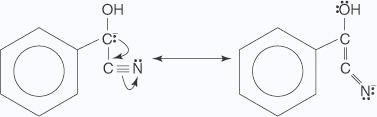
- The carbanion attacks a second molecule of benzaldehyde
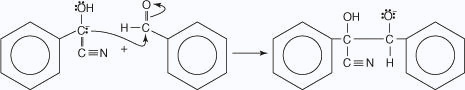
- The alkoxide ion removes a proton from the hydroxide group.
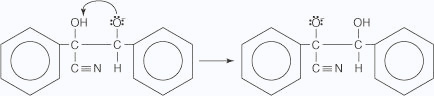
- A pair of electrons on the alkoxide ion are attracted to the carbon bonded to
the cyanide group,
which then leaves to generate the product.
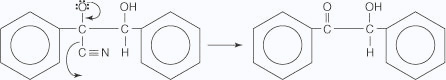
- In carbonyl - carbonyl condensation reaction, two carbonyl molecules react to form a single organic product together with a molecule of water.
- Reactions of two molecules of different aldehydes or ketones are called as
cross aldol
condensation -a mixture of products is formed except under certain
conditions
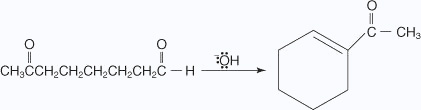
Cross-aldol condensation When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation. If both of them contain a-hydrogen atoms, it gives a mixture of four products. Ketones can also be used as one component in the cross aldol reactions. To be useful, a cross-aldol must be run between an aldehyde possessing an α hydrogen and a second aldehyde that does not have α hydrogens.
Reduction to alcohols: Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4) as well as by catalytic hydrogenation. Their structures are:
The reduction of an aldehyde The same organic product is obtained either by using lithium tetrahydridoaluminate or sodium tetrahydridoborate. Example, with ethanal you get ethanol:

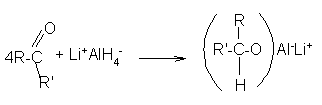
The product is then treated with a dilute acid (such as dilute sulphuric acid or dilute hydrochloric acid) to release the alcohol from the complex ion.
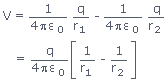

The reduction of a ketone: Again the product is the same whichever of the two reducing agents you use. Example, with propanone you get propan-2-ol:
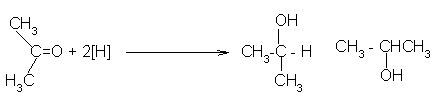
- Using sodium tetrahydridoborate (sodium borohydride)
Solid sodium tetrahydridoborate is added to a solution of the aldehyde or ketone
in an alcohol such as methanol,
ethanol or propan-2-ol. Depending on which recipe , it is either heated under
reflux or left for some
time around room temperature. This almost certainly varies depending on the
nature of the aldehyde or ketone.
At the end of this time, a complex similar to the previous one is formed.
In the second stage of the reaction, water is added and the mixture is boiled to release the alcohol from the complex.
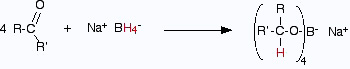 Again, the alcohol formed can be recovered from the mixture by fractional distillation.
Again, the alcohol formed can be recovered from the mixture by fractional distillation.
- Reduction to hydrocarbons: The carbonyl group of aldehydes and
ketones is reduced to CH2 group.
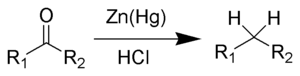
- Clemmensen reduction is a chemical reaction described
as a reduction of ketones (or aldehydes)
to alkanes using zinc amalgam and hydrochloric acid. This reaction is
named after Erik Christian Clemmensen,
a Danish chemist.
The Clemmensen reduction is particularly effective at reducing aryl-alkyl ketones. With aliphatic or cyclic ketones, zinc metal reduction is much more effective. The substrate must be stable in the strongly acidic conditions of the Clemmensen reduction. As a result of Clemmensen Reduction, the carbon of the carbonyl group involved is converted from sp2 hybridization to sp3 hybridization. The oxygen atom is lost in the form of one molecule of water. Acid sensitive substrates should be reacted in the Wolff-Kishner reduction, which utilizes strongly basic conditions.
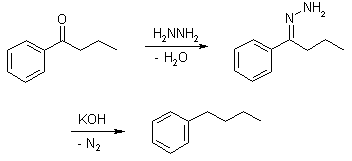
- Wolff-kishner reduction The reduction of aldehydes and ketones to alkanes. In Wolff-kishner reaction Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
- Clemmensen reduction is a chemical reaction described
as a reduction of ketones (or aldehydes)
to alkanes using zinc amalgam and hydrochloric acid. This reaction is
named after Erik Christian Clemmensen,
a Danish chemist.
Aldehydes can be oxidized to carboxylic acid with both mild and strong oxidizing agents. However, ketones can be oxidized to various types of compounds only by using extremely strong oxidizing agents. Ketones are generally oxidized under vigorous conditions, i.e.,strong oxidizing agents and at elevated temperatures. Their oxidation involves carbon-carbon bond cleavage to afford a mixture of carboxylic acids having lesser number of carbon atoms than the parent ketone. Typical oxidizing agents for aldehydes include either potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7) in acid solution and Tollens reagent. Peroxy acids, such as peroxybenzoic acid are used to oxidize ketones.
- Benzaldehyde is oxidized to benzoic acid by KMnO4 oxidizing agent.
- Cyclohexane carbaldehyde is oxidized to Cyclohexanoic acid by Tollen's reagent.
- Cyclohexyl methyl ketone is oxidized to cyclohexylacetate by using peroxybenzoic acid as oxidizing agent.
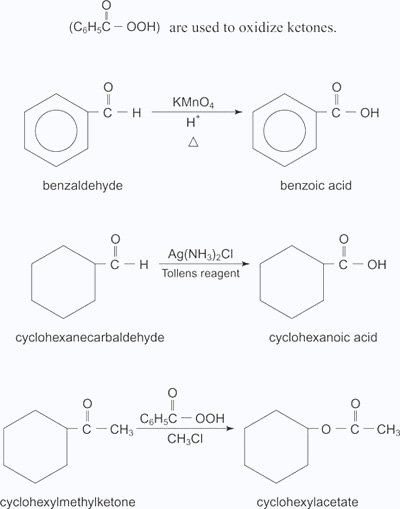
Baeyer-Villiger oxidation is a ketone oxidation, and it requires the extremely strong oxidizing agent peroxybenzoic acid. For example, peroxybenzoic acid oxidizes phenyl methyl ketone to phenyl acetate (an ester).
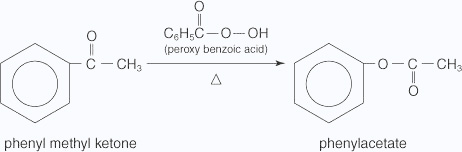
Haloform reaction: In haloform reaction Aldehydes and ketones having one methyl group linked to a carbonyl carbon atom (i.e )methyl ketone are oxidized by sodium hypohalite to sodium salts of corresponding carboxylic acids having 1 carbon atom less than that of carbonyl compound.

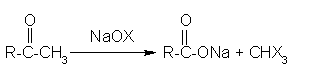
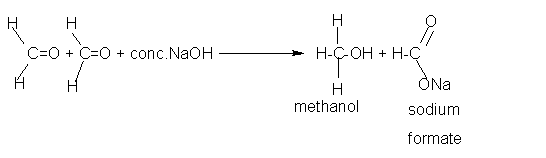
Cannizzaro reaction: Aldehydes which do not have α hydrogen atom react with concentrated sodium hydroxide (NaOH) or potassium hydroxide (KOH) in such a way that one molecule get oxidized to acid and the second molecule gets reduced to alcohol. Note two molecules of aldehyde participates in the reaction. This self oxidation-reduction under the influence of a base is known as the Cannizzaro's reaction.
- Formaldehyde does not possess a-hydrogen atom and therefore undergoes
Cannizzaro's reaction.
Acetaldehyde does not give this reaction.
Formaldehyde(HCHO) two molecules + warm NaOH give Methanol (CH3OH) and Sodium Formate (CHOONa) CH3OH is the reduction product HCHO becomes CH3OH (two hydrogen atoms are getting in). CHOONa is the oxidation product. one 'H' has gone out and One 'O' came in along with Na.
- Benzaldehyde
Electrophilic substitution reaction: Aromatic aldehydes and ketones undergo electrophilic substitution at the ring in which the carbonyl group acts as a deactivating and meta-directing group.