Many of the reactions that occur in organic chemistry are either acid–base reactions themselves, or they involve an acid–base reaction at some stage. In general, both acids and bases are electrolytes, and they dissolve in water to give solutions that conduct electricity.
An electrolyte is a substance, which dissociates in a solvent to form an ionic solution. Strong electrolytes dissociate almost completely. Weak electrolytes form an equilibrium between the associated and dissociated forms and are only partly dissociated.

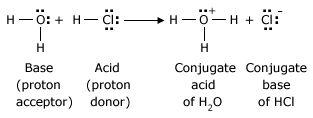
The Bronsted–Lowry Definition of Acids and Bases

Lewis Definition of Acids and Bases
In the same year as Bronsted and Lowry, Lewis proposed an even more general definition based on the sharing of electron pairs: an acid is able to accept a pair of electrons to form a new bond; a base is able to donate a pair of electrons to form a new bond. Aluminum chloride, for example reacts with ammonia in the same way that a proton does.
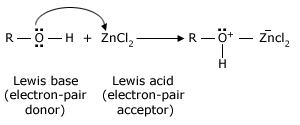
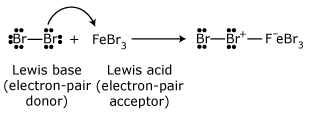
A Lewis acid needs an empty valence orbital for the electron pair from the base to go into. Metal cations like Cu2+ attract electrons because of their positive charge and have at least one empty orbital. Molecules with an unfilled octet, like BF3, are strong Lewis acids. Some neutral molecules such as the oxides of non–metals are also Lewis acids. One example of this is carbon dioxide.
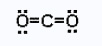
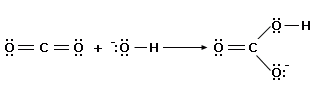

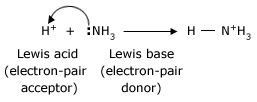
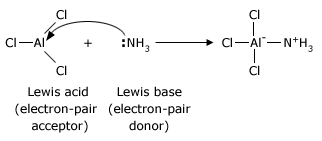
Bases are much the same in the Lewis theory and the Bronsted–Lowry theory, because in the Bronsted–Lowry theory a base must donate a pair of electrons in order to accept a proton. The Lewis theory, by virtue of its broader definition of acids, allows acid–base theory to include all of the Bronsted – Lowry reactions and a great many others.
Any electron–deficient atom can act as a Lewis acid. Many compounds containing Group 3A elements such as boron and aluminum are Lewis acids because Group 3A atoms have only a sextet of electrons in their outer shell. Many other compounds that have atoms with vacant orbitals also act as Lewis acids. Zinc and iron(III) halides (ferric halides) are frequently used as Lewis acids in organic reactions. Two examples that we shall study later are the following:
Amphiprotism Definition
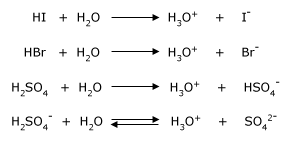
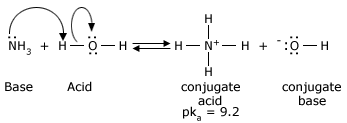
A prophylactic (also called amphoteric) substance is capable of donating or accepting protons, depending on the reaction conditions. Water is the most common amphoteric species, but not the only one. Liquid ammonia (NH3) is also amphiprotic, as are the anions of polyprotic acids. Consider how a substance can both accept and donate an electron pair. A polyprotic acid is one, which has more than one proton to donate (eg: H2SO4). It is an amphiprotic substance as well.
Strength of Acids and Bases
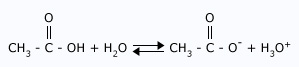
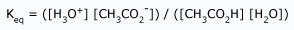
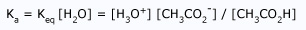

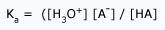
Acidity Negative Logarithm
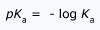

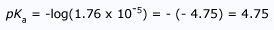
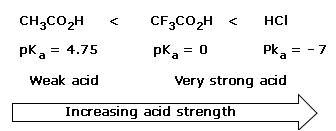
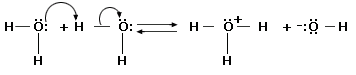
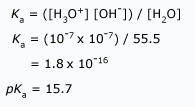
Predicting the Strength of Bases
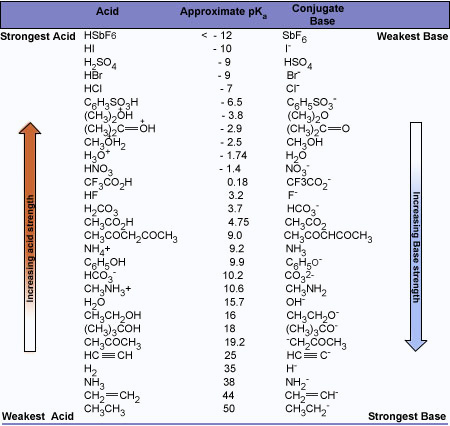
There is a principle that allows us to estimate the strengths of bases. Simply stated, the principle is this:The stronger the acid, the weaker will be its conjugate base.
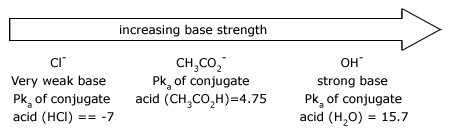
Trends in acidity
The strength of an acid depends on the extent to which a proton can be separated from it and transferred to a base. Removing the proton involves breaking a bond to the proton, and it involves making the conjugate base more electrically negative. When we compare compounds in a vertical column of the, periodic table, the strength of the bond to the proton is the dominating effect. The acidities of the hydrogen halides furnish an example:
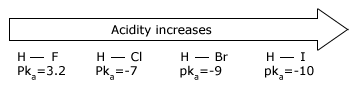
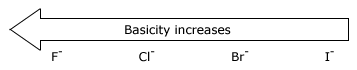

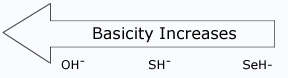
Acidity increases from left to right when we compare compounds in the same horizontal row of the periodic table.Bond strengths are roughly the, same, and the dominant factor becomes the electro negativity of the atom bonded to the hydrogen. The electronegativity of this atom affects acidity in two related ways. It affects the polarity of the bond to the proton and it affects the relative stability of the anion (conjugate base) that forms when the proton is lost. Let us compare two hypothetical acids, H–A and H–B.

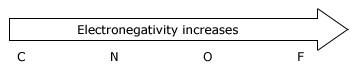
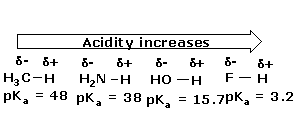
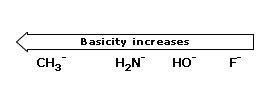
Energy changes
Chemical energy is a form of potential energy. It exists because attractive and repulsive electrical forces exist between different pieces of the molecules. Nuclei attract electrons, nuclei repel each other, and electrons repel each other.
Potential Energy and Covalent Bonds
Atoms and molecules possess potential energy – often called chemical energy, that can be released as heat when they react. Because heat is associated with molecular motion, this release of heat results from a change from potential energy to kinetic energy.

A convenient way to represent the relative potential energies of molecules is in terms of their relative enthalpies or heat contents, H. (Enthalpy comes from en + Gk: thalpein to heat.) The difference in relative enthalpies of reactants and products in a chemical change is called the enthalpy change and is symbolized by ΔH°. [The δ (delta) in front of a quantity usually means the difference, or change, in the quantity. The superscript ° indicates that the measurement is made under standard conditions.] By convention, the sign of ΔH° for exothermic reactions (those evolving heat) is negative. Endothermic reactions (those that absorb heat) have a positive ΔH°. The heat of reaction, ΔH°, measures the change in enthalpy of the atoms of the reactants as they are converted to products. For an exothermic reaction the atoms have a smaller enthalpy as products than they do as reactants. For endothermic reactions, the reverse is true.