Other preparation methods for chloroform
From acetone (from propanone):
When acetone is heated with bleaching powder, or with chlorine and alkali,
the following reactions take place.
- Reaction of chlorine with acetone producing trichloroacetone.
- Trichloroacetone is decomposed by either calcium hydroxide or an alkali, to give chloroform.
- By partial reduction of carbon tetrachloride

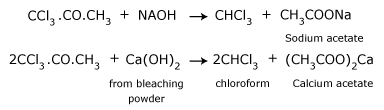
The net reaction when bleaching powder is used is,
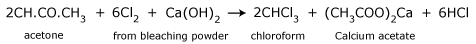
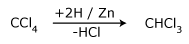
Zinc and hydrochloric acid is taken and shaken at a slightly elevated
temperature.
Manufacture of Chloroform:
Chloroform is generally
manufactured by the following methods.
- From methane: Chloroform can be manufactured by chlorinating methane. A mixture of CH4 : Cl2 : N2 in the ratio 8 : 3 : 80 is passed over a partially reduced sample of cupric chloride. Chloroform is recovered by distillation.
- From carbon tetrachloride: Chloroform can also be prepared on a large scale by partial reduction of carbon tetrachloride using iron filings and water.
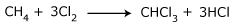
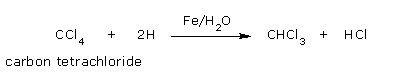
Iodoform
Preparation of Iodoform:
Iodoform is obtained by the
action of iodine on ethanol (ethyl alcohol),
or on propanone (acetone) in the presence of an alkali. This reaction is called haloform
reaction, and is commonly known as iodoform test.
- From Ethanol:
- From acetone:
- when ethanol is used:
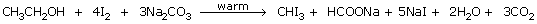
- when propanone (acetone) is used:
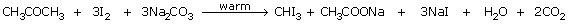
When ethanol is warmed (60°C) with iodine in the presence of an alkali (NaOH, or
Na2CO3), iodoform is obtained.
Step 1:
NaOH + I2
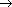
 NaI + H2O
NaI + H2O
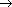
 NaI + H2O
NaI + H2O
Step 2:
Oxidation of ethanol to ethanal (acetaldehyde).
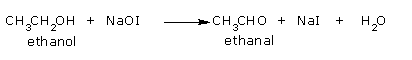
Step 3:
Ethanol so formed undergoes iodination.
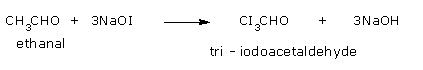
Step 4:
Hydrolysis (cleavage) of Cl3CHO by alkali.
Cl3CHO + NaOH
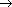
 +
HCOONa
+
HCOONa
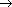
 +
HCOONa
+
HCOONa
Net reaction:
C2H5OH + 4I2 +
6NaOH
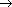 CHI3 + HCOONa + 2NaI +
5H2O
CHI3 + HCOONa + 2NaI +
5H2O
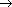 CHI3 + HCOONa + 2NaI +
5H2O
CHI3 + HCOONa + 2NaI +
5H2O
Iodoform can also be prepared by warming acetone with iodine in the presence of an
alkali. The reactions are
Step 1:
2NaOH + I2
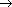 NaOI +
NaI + H2O
NaOI +
NaI + H2O
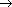 NaOI +
NaI + H2O
NaOI +
NaI + H2O
Step 2:
Iodination of acetone.
CH3.CO.CH3 + 3NaOI 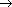
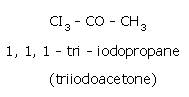 + 3NaOH
+ 3NaOH
Step 3:
Hydrolysis (cleavage) of Cl3.CO.CH3
by alkali.
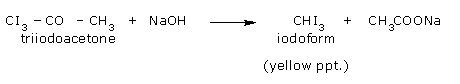
Net reaction:
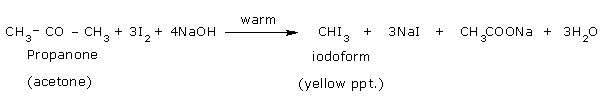
The reactions for the preparation of iodoform when Na2CO3 is
used in place of NaOH are as follows:
Physical Properties of Iodoform:
- Iodoform is a yellow crystalline solid (hexagonal plates) with characteristic unpleasant odor. It melts at 392 K, (119°C).
- Iodoform is insoluble in water, but soluble in alcohol, chloroform and ether.
- It has antiseptic properties which is due to the liberation of iodine from it.
Chemical Properties of Iodoform:
Iodoform shows
chemical behavior similar to that of chloroform, but it is less stable.
Some of the typical reactions given by iodoform are:
- Stability
On heating, iodoform decomposes to give iodine vapor. Due to the liberation of free iodine iodoform shows antiseptic action. Moisture, air and light also cause the decomposition of iodoform.
- Hydrolysis
On boiling with alcoholic sodium hydroxide, iodoform gives sodium formate.

- Heating with silver powder
When heated with Ag powder, iodoform gives ethyne (acetylene).

- With primary amines
Carbylamine reaction. Iodoform when heated with a primary amine and alcoholic potash, gives an offensive smelling compound known as carbylamine.

- With silver nitrate
Iodoform gives yellow colored precipitate of AgI when heated with alcoholic silver nitrate.
Uses of Iodoform:
Iodoform is used as an antiseptic. Its antiseptic property is due to the liberated iodine. However, due
to
its unpleasant smell, it has been replaced by other formulations containing iodine. It is used in the
manufacture of certain pharmaceuticals.
Carbon tetrachloride(CCl4)
Preparation of Carbon tetrachloride:
Carbon tetrachloride
can be prepared on the industrial scale by the following methods.
- From methane:
- From carbon disulphide:
- From propane:
By the chlorination of methane in the presence of sunlight.
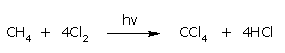
Carbon tetrachloride can be obtained by the action of chlorine on carbon disulphide
in the presence of aluminum chloride as a catalyst.
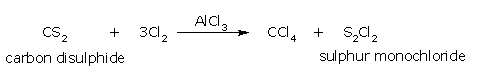
Propane reacts with chlorine at 400°C and 70-100 atm pressure to give
CCl4,
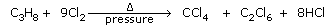
Physical Properties of Carbon Tetrachloride:
- Carbon tetrachloride is a colorless heavy liquid (b.p. 350 K, (77°C))
- It is insoluble in water but soluble in organic solvents.
- It is a good solvent for oils, fats and resins.
- It is non-inflammable.
- It is stable at red heat (
 500°C).
500°C).
In past carbon tetrachloride was used as a fire extinguishing material,
under the name pyrene.
When a fine spray of liquid CCl4 is directed at the fire, it vaporizes. The dense
vapor of CCl4
cut off the supply of air to the burning article. As a result, the fire gets extinguished.
However, at higher temperature in the presence of steam, carbon tetrachloride gets
oxidized to phosgene.
So, care should be taken while using carbon tetrachloride in fire fighting operations.
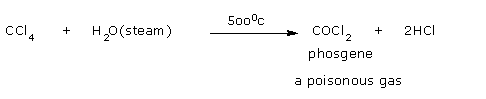
Chemical Properties of Carbon Tetrachloride:
- Reduction
Carbon tetrachloride on reduction with moist iron filings gives chloroform.
- Hydrolysis
On boiling with alcoholic sodium hydroxide, iodoform gives sodium formate.
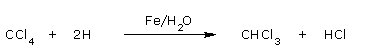
- Hydrolysis
In the presence of steam, CCl4 gives phosgene.
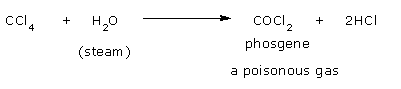
- Formation of Freon
It reacts with SbF3 in presence of SbCl5 to give freon.
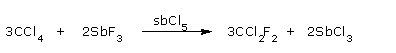
Uses:
- It is used as a fire extinguisher under the name Pyrene
- It is used as a solvent for fats, oils, waxes, greases, resins etc and in dry cleaning
- In medicines for the estimation of hook worms
- It is used for manufacture of freon and chloroform