Chain growth polymerization
Chain growth polymerization proceeds in three important steps :
- Initiation step:
- Propagation step:
- Termination step:
This step involves the formation of a reactive particle.
It consists of a growing polymer chain having reactive particles.
In this step, the growth of the chain is finally terminated.
In the free radical polymerization , monomer is activated by the action of light, heat, or by adding
chemicals, known as initiators.
Examples of initiators are benzoyl per-oxide and azo bisisobutyronitrile (AIBN).
The polymerization of styrene, initiated by benzoyl peroxide, is a typical example of free radical
polymerization.
- Initiation:
- Propagation:
- Termination:
- Recombination of free radicals:
- Reaction with inhibitors:
- Reaction with the solvent:
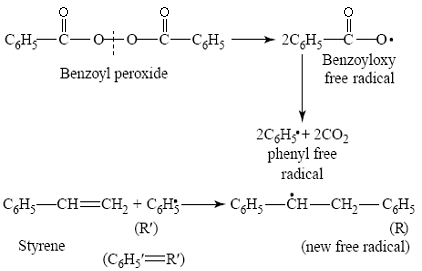
This consists of the decomposition of benzoyl peroxide into benzoyl oxy-free
radicals
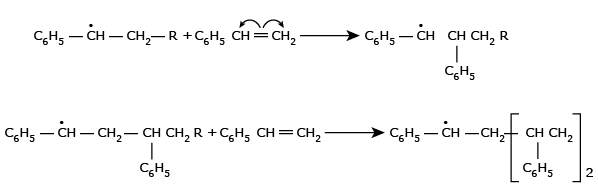
In the extremely rapid chain-propagating step, the new free radical adds to the
double bond of another
styrene monomer and forms a new radical which is capable of further interacting with
the initial styrene
monomers and in this step macro–free radical is formed.
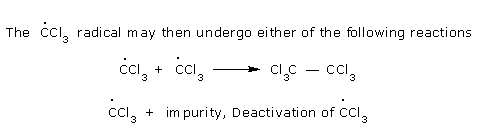
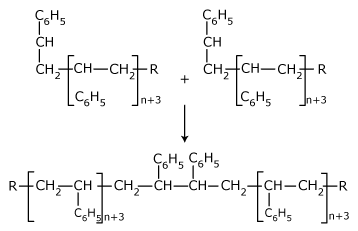
The macro-free radicals are deactivated by one of the
following methods.
The growing free radical
reacts with the other growing free radical.
Polymeric chain can be
terminated by the reaction of inhibitors,
such as hydroquinone, phenol, amines, etc. For example, phenoxy
radicals(ArO°) derived from phenol (ArOH)
are highly resonance stabilized and so unreactive that they cannot initiate
chain reaction and thus,
polymeric chain is terminated

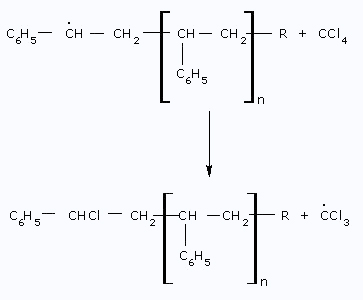
The solvent molecule, such as
CCl4, reacts
with the Radical and produces CCl3 radical.