Structures of compounds with different co-ordination numbers
Coordination complexes exists in certain geometrical forms depending on their coordination numbers. I.e number of ligand atoms attached to the central ion /metal ion.
The following are some of the geometrical forms of the complexes depending on the
coordination numbers.
Coordination number 2
Linear geometry
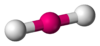
Examples of discrete (finite) complex:
[Ag(CN)2]-
in
KAg(CN)2 ,Ag in silver cyanide,
Examples in crystals: Au in AuI
Coordination number 3
Trigonal planar geometry
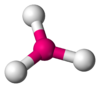 Examples of discrete (finite) complex :
Cu(CN)32- in Na2Cu(CN)3.3H2O
Examples of discrete (finite) complex :
Cu(CN)32- in Na2Cu(CN)3.3H2O
Examples in crystals : O in TiO2 rutile structure
Coordination number 4
Tetrahedral planar geometry
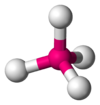 Examples of discrete (finite) complex : CoCl42-
Examples of discrete (finite) complex : CoCl42-
Examples in
crystals:Zn and S in zinc sulfide, Si in silicon dioxide
Coordination number 5
Square planar geometry
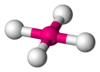 Examples of discrete (finite) complex :
AgF4-
Examples of discrete (finite) complex :
AgF4-
Examples in crystals:CuO
Coordination number 5
Trigonal
bipyramidal planar geometry
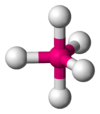 Examples of discrete (finite) complex :
SnCl5-
Examples of discrete (finite) complex :
SnCl5-
Coordination number 6
Square pyramidal geometry
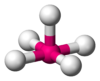 Examples of discrete (finite) complex :
InCl5 2-(Net4)2InCl5
Examples of discrete (finite) complex :
InCl5 2-(Net4)2InCl5
Coordination number 6
Octahedral geometry
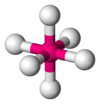 Examples of discrete (finite) complex : Fe(H2O)62+
Examples of discrete (finite) complex : Fe(H2O)62+
Examples in crystals:Na and Cl in NaCl
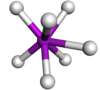
Coordination number 7
Trigonal
prismatic geometry
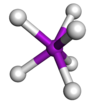 Examples of discrete (finite) complex :
Mo(SCHCHS)3
Examples of discrete (finite) complex :
Mo(SCHCHS)3
Examples in crystals:As in NiAs, Mo in Mo(SCHCHS)3
Coordination number 7
Pentagonal bipyramidal geometry
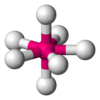 Examples of discrete (finite) complex :
ZrF73- in (NH4)3ZrF7
Examples of discrete (finite) complex :
ZrF73- in (NH4)3ZrF7
Examples in
crystals : PaCl5
Coordination number 7
Face capped octahedral geometry
 Examples of discrete (finite) complex
:[HoIII(PhCOCHCOPh)3(H2O)]
Examples of discrete (finite) complex
:[HoIII(PhCOCHCOPh)3(H2O)]
Examples in crystals: La in
A - La2O3
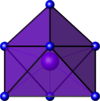
Coordination number 8
Trigonal
prismatic geometry, square face monocapped geometry
 Examples of discrete (finite) complex
:TaF72- in K2TaF7
Examples of discrete (finite) complex
:TaF72- in K2TaF7
Coordination number 8
Cubic geometry
Examples of discrete (finite) complex Caesium chloride, calcium
fluoride
Coordination number 8
Square
antiprism geometry
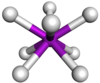
Examples of discrete (finite) complex
TaF83- in Na3TaF8
Examples in crystals
:Thorium(IV) iodide
Coordination number 8
Dodecahedral geometry
(note whilst this is term generally used the correct term is
bisdisphenoidor snub disphenoid as this polyhedron is a deltahedron)
 Examples of discrete
(finite) complex Mo(CN)84- in
K4[Mo(CN)8].2H2O
Examples of discrete
(finite) complex Mo(CN)84- in
K4[Mo(CN)8].2H2O
Examples in crystals :Zr in
K2ZrF6
Coordination number 8
Hexagonal bipyramidal geometry
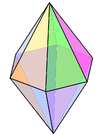 Examples of discrete (finite) complex N in
Li3N
Examples of discrete (finite) complex N in
Li3N
Coordination number 8
Octahedral geometry
Examples of discrete (finite) complex trans-bicapped Ni in nickel arsenide,
NiAs;
6 As neighbours + 2 Ni capping
Coordination number 8
Trigonal prismatic
geometry, Triangular face bicapped geometry
Examples of discrete (finite) complex: Ca
in CaFe2O4 square face bicapped geometry,
Examples of discrete
(finite) complex PuBr3
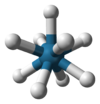
Coordination number 9
Trigonal
prismatic geometry, Square face tricapped
Examples of discrete (finite) complex
[ReH9]2- in potassium nonahydridorhenate ,
SrCl2.6H2O,
 Examples in crystals Th in
RbTh3F13
Examples in crystals Th in
RbTh3F13
Coordination number 9
Monocapped
square antiprismatic geometry,

Examples of discrete (finite) complex
[Th(tropolonate)4(H2O)]
Examples in crystals La in LaTe2
Examples in crystals La in LaTe2
Coordination number 10
Bicapped square
antiprismatic geometry,
Examples of discrete (finite) complex
Th(C2O4)42-
Coordination number 11
Examples of discrete (finite) complex Th in [ThIV(NO3)4(H2O)3] (NO3)- is bidentateCoordination number 12
Icosahedron geometry

Examples of discrete
(finite) complex Th in Th(NO3)62- ion in
Mg[Th(NO3)6].8H2O
Coordination number 12
Cuboctahedron
geometry

Examples of
discrete (finite) complex ZrIV(n3- (BH4)4) atoms
Coordination number 12
Anticuboctahedron
geometry,
Triangular orthobicupola geometry

Coordination number 14
Bicapped hexagonal
geometry
Examples of discrete (finite) complex :antiprismatic
U(BH4)4