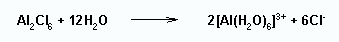Anhydrous aluminum trichloride AlCl3 is probably the most commonly used Lewis acid and also one of the most powerful. It finds widespread application in the chemical industry as a catalyst for Friedel–Crafts reactions, both acylations and alkylations. Important products are detergents and ethylbenzene. It also finds use in polymerization and isomerization reactions of hydrocarbons. The Friedel–Crafts reaction is the major use for aluminum chloride, for example in the preparation of anthraquinone (for the dye stuffs industry) from benzene and phosgene.In the general Friedel–Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown.

The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding because the reaction is more sluggish. For both reactions, the aluminum chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed. A general problem with the Friedel–Crafts reaction is that the aluminum chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. This complication sometimes generates a large amount of corrosive waste. For these and similar reasons, more recyclable or environmentally benign catalysts have been sought. Thus, the use of aluminum trichloride in some applications is being displaced by zeolites.
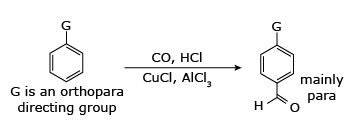
Aluminum chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann–Koch reaction which uses carbon monoxide,hydrogen chloride and a copper(I) chloride co-catalyst.
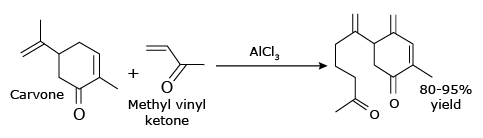
Aluminum chloride finds a wide variety of other applications in organic chemistry.For example, it can catalyze the "ene reaction", such as the addition of 3–buten–2–one(methyl vinyl ketone) to carvone:
AlCl3 is also widely used for polymerization and isomerization reactions of hydrocarbons. Important examples include the manufacture of ethylbenzene, which used to make styrene and thus polystyrene, and also production of dodecylbenzene, which is used for making detergents.
Aluminum chloride combined with aluminum in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g.bis(benzene)chromium, from certain metal halides via the so-called Fischer–Hafner synthesis.
Hydrated aluminum chloridesThe hexahydrate has few applications, but aluminum chlorohydrate is a common component in antiperspirants at low concentrations.Hyperhidrosis sufferers need a much higher concentration (12% or higher), sold under such brand names as Drysol, sunsola, Maxim, Odaban, CertainDri, B+Drier, Anhydrol Forte and Driclor.
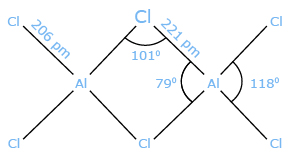
Structure of Aluminum chloride AlCl3 adopts three different structures, depending on the temperature and the state (solid, liquid, gas). Solid AlCl3 is a sheet-like layered cubic close packed layers. In this framework, the Al centers exhibit octahedral coordination geometry.In the melt, aluminum trichloride exists as the dimer Al2Cl6, with tetracoordinate aluminum. This change in structure is related to the lower density of the liquid phase (1.78 g/cm3) Vs solid aluminum trichloride (2.48 g/cm3). Al2Cl6 dimers are also found in the vapor phase. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3, which is structurally analogous to BF3. The melt conducts electricity poorly,unlike more ionic halides such as sodium chloride.
When dissolved in water the dimer breaks up to give free ions.