This cell has replaced Castner–Kellner cell to some extent. Kellner–Solvay cell consists of a rectangular trough, at the base of which there is a thin layer of mercury which flows from one end to the other acting as a cathode. A concentrated brine solution flows slowly through the cell in the same direction as the mercury and is maintained at a constant level.
Mercury so produced is sent back to the cell. The spent brine solution leaving the cell is saturated with more common salt and returned to the cell.
Using a diaphragm cell: In this method, sodium hydroxide is obtained from brine (20% NaCl solution) by electrolysis. The reactions are
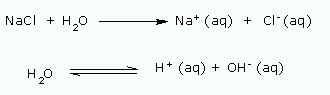
At anode:
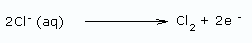
At cathode:
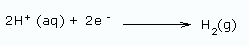
In this method, care is taken not to allow NaOH and Cl2 to come in contact with each other. This is done to prevent the formation of sodium hypochlorite.
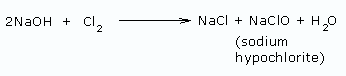
In modern cells, the diaphragm is replaced by a Nafion membrane.
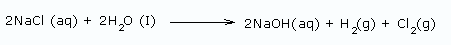
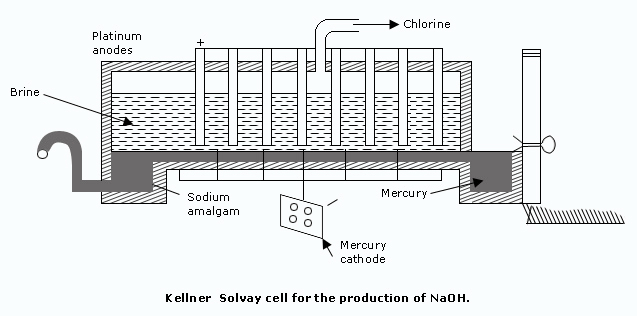
The Kellner–Solvay cell

The Nelson cell for the manufacture of sodium hydroxide, chlorine and hydrogen gases are valuable by–products.