While observing the time taken by chemicals to react with each other, we will observe that some reactions are slow and some are fast. Some chemical reactions, such as the rusting of iron, are slow, while others, such as the burning of gasoline, are fast.

Chemical reactions therefore have a certain rate at which they proceed. The rate of a chemical reaction can be defined as the change in the concentration of reactants or products in a unit time.
| When we write down a balanced chemical reaction, we have seen earlier that it does not tell you anything about, |
| 1.whether the reaction is actually possible |
| 2.whether the reaction is complete |
| 3.the rate of the reaction |
| 4.the energy changes in the reaction. |
| When the rate of reactions is taken into account, the lack of information in a balanced chemical equation, is taken care of |
| The rate of a chemical reaction depends on several factors. Some of them are given below: |
| 1.Type or Nature of Reactants |
| 2.The Particle Size |
| 3.Temperature of Reaction |
| 4.Concentration of the Reactants |
| 5.Presence of a Catalyst |
Type or Nature of Reactants
Every chemical has specific properties. Therefore the rate of reaction varies with the particular chemical used. Thus the rate of reaction of HCl on CuSO4 will be different from that of HCl on FeSO4. Some elements, because of the way their electronic configuration occurs, are very reactive. For example Na, K, Rb readily give off their electrons. F, Cl, Br are elements which quickly accept electrons. On the other hand noble gases like He, Ne, Ar, Kr are chemically inert. Non – metals like C, N, O react but not as vigorously as F, Cl, Br, etc. In most cases valence of the reactants will give you a rough estimate about the reaction rates.
Examples of fast reactions
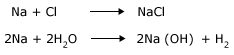
Examples of slow reactions
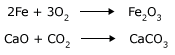
Particle size
It is generally observed that when a chemical is used in the form of a fine powder the reactions go fast, but not when big lumps or large crystals are used. The reason for this is very simple. In a chemical reaction, each substance is changing at the atomic level. In smaller particles, the surface area for reaction on the microscopic level is larger. In other words, if the number of collisions between the reacting molecules is large, the reaction rate would be higher. Here is an example to see the difference in rates of reaction with respect to the particle size
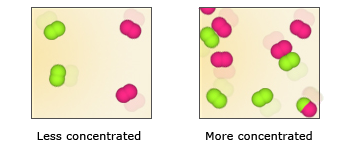
Take two test tubes. In one put some iron filings and in the other a piece of iron like a nail. Let the weight of the iron filings and the nail be the same. Pour some hydrochloric acid into both the test tubes. Watch the reactions. You will observe that the iron filings dissolve much faster than the nail. The reaction that is taking place is,
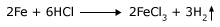
In another activity, take a piece of marble (CaCO3) in a test tube. In another put some powdered CaCO3. Take equal quantities by weight in both the test tubes. Add HNO3 in both the test tubes. In both the test tubes the reaction starts, but in the case of the powder you will see the powder disappear quickly. The reaction that is taking place is
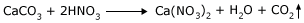
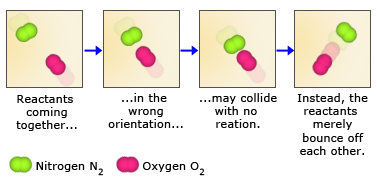

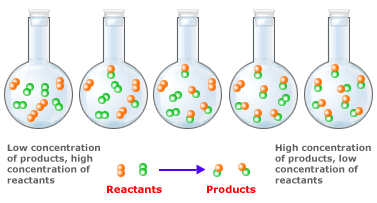
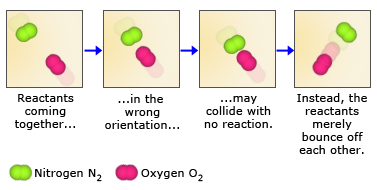
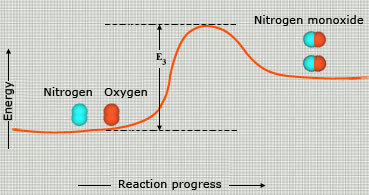
Because reactant molecules must collide in order for a reaction to occur, increasing the number of collisions can increase the rate of a reaction. An effective way to increase the number of collisions is to increase the concentration of the reactants. Higher concentrations, there are more molecules in a given volume, which makes collisions between molecules more probable. As an analogy, consider a bunch of people on a dance floor–as the number of people increases, so does the rate at which they bump into one another. An increase in the concentration of nitrogen and oxygen molecules, therefore, leads to a greater number of collisions between these molecules and hence a greater number of nitrogen monoxide molecules formed in a given period of time.
Not all collisions between reactant molecules lead to products, however, because the molecules must collide in a certain orientation in order to react. Nitrogen and oxygen, for example, are much more likely to form nitrogen monoxide when the molecules collide in a parallel fashion. When they collide in a perpendicular fashion, nitrogen monoxide does not form. For larger molecules, which can have numerous orientations, this orientation requirement is even more restrictive.
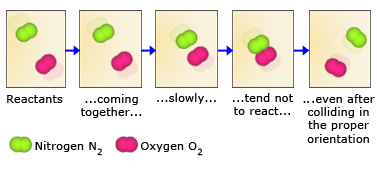
The second reason for not all collisions lead to product formation is that the reactant molecules must also collide with enough kinetic energy to break their bonds. Only then is it possible for the atoms in the reactant molecules to change bonding partners and form product molecules. The bonds in N2 and O2 molecules, for example, are quite strong. For these bonds to be broken, collisions between the molecules must contain enough energy. As a result, collisions between slow – moving N2 and O2 molecules, even those that collide in the proper orientation, may not form NO.
Temperature of reaction
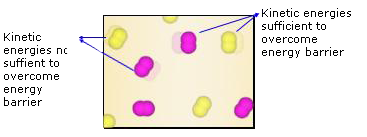
Some reactions go faster if the temperature is increased. This again is because of the fact that higher the temperature more the number of collisions. The higher the temperature of a material, the faster its molecules are moving and the more forceful the collisions between them. Higher temperatures, therefore, tend to increase reaction rates. The nitrogen and oxygen molecules that make up our atmosphere, for example, are always colliding with one another. At the ambient temperatures of our atmosphere, however, these molecules do not generally have sufficient kinetic energy to allow for the formation of nitrogen monoxide.
The heat of a lightning bolt, however, dramatically increases the kinetic energy of these molecules, to the point that a large portion of the collisions in the vicinity of the bolt result in the formation of nitrogen monoxide. As discussed earlier, the nitrogen monoxide formed in this manner undergoes further atmospheric reactions to form chemicals known as nitrates that plants depend on to survive. This is an example of nitrogen fixation. The energy required to break bonds can also come from the absorption of electromagnetic radiation. As the radiation is absorbed by reactant molecules, the atoms in the molecules may start to vibrate so rapidly that the bonds between them are easily broken. In many instances, the direct absorption of electromagnetic radiation is all it takes to break chemical bonds and initiate a chemical reaction. The common atmospheric pollutant nitrogen dioxide, NO2, may transform to nitrogen monoxide and atomic oxygen merely upon exposure to sunlight: At any given temperature, there is a wide distribution of kinetic energies in reactant molecules. Some are moving slowly and others quickly. The temperature of a material is
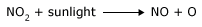
Simply the average of all these kinetic energies. The few fast – moving reactant molecules in the figure are the first to transform to product molecules because these are the molecules that have enough energy to pass over the energy barrier.
Fast – moving reactant molecules possess sufficient energy to pass over the energy barrier and hence are the first ones to transform to product molecules.When the temperature of reactants is increased, the number of reactant molecules having sufficient energy to pass over the barrier also increases, which is why reactions are generally faster at higher temperatures. Conversely, at lower temperatures, there are fewer molecules having sufficient energy to pass over the barrier, which is why reactions are generally slower at lower temperatures.In the reaction between Fe and HCl, heat the HCl and pour over iron fillings.
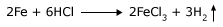
You will observe that the reaction is faster than before. This indicates that the reaction is faster when the temperature is higher. It is easy to see why, as the temperature rises, the atoms or molecules of the reactant will start jittering around more. This will increase the probability of collision between the reacting atoms or molecules. As the probability increases, the reaction rate will increase, too. Most chemical reactions are influenced by temperature, including those reactions occurring in living bodies. The body temperature of animals that regulate their internal temperature, such as humans, is fairly constant. However, the body temperature of some animals, such as the alligator, rises and falls with the temperature of the environment. On a warm day, the chemical reactions occurring in an alligator are –up to speed,– and the animal can be most active. On a chilly day, however, the chemical reactions proceed at a lower rate, and as a consequence, the alligator's movements are unavoidably sluggish.

This alligator became immobilized on the pavement after being caught in the cold night air. By mid – morning,shown here, the temperature had warmed sufficiently to allow the alligator to get up and walk away.
Concentration of Reactants
More the concentration of the reactants, faster is the rate of the reaction, because again better are the chances of collision. If one puts dilute HCl on CaCO3 powder the rate of the reaction (indicated by the bubbles of the gas being evolved) is much slower than when concentrated HCl is used.
For example in the following reactions:
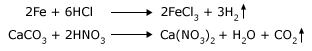
Observe the difference in time of reaction when you use dilute and concentrated acids. The reactions will be much faster in case of concentrated acids. In other words, larger the concentration of the reactants, larger will be the probability of the reactants coming together, hence faster would be the reaction rates. In case of reacting gases, the gas pressure will be a parameter in place of concentration. Higher the gas pressure faster is the reaction.
Presence of a Catalyst
Catalysts are compounds, which improve the rate of the reaction but they themselves remain unchanged at the end of the reaction. They assist the reaction in one direction or the other. For example if you heat KClO3, it will start decomposing at a very high temperature. If a bit of MnO2 is added, the decomposition of KClO3 is faster and at a lower temperature. Chlorophyll in leaves acts as a catalyst for the plants to convert energy from the sun to food. Platinum is used as a catalyst for reactions where acids are manufactured.
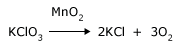
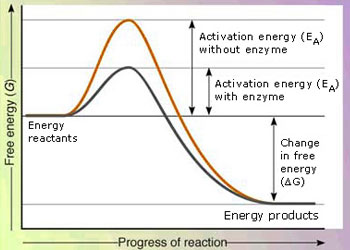
To appreciate the need for a catalyst in a reaction, we have to be introduced to the idea of activation energy, in a reaction. Whether the result of collisions, absorption of electromagnetic radiation, or both, broken bonds are a necessary first step in most chemical reactions. The energy required for this initial breaking of bonds can be viewed as an energy barrier. The minimum energy required to overcome this energy barrier is known as the activation energy Ea. In the reaction between nitrogen and oxygen to form nitrogen monoxide, the energy barrier is so high (because the bonds in N2 and O2 are strong) that only the fastest – moving nitrogen and oxygen molecules possess sufficient energy to react. Figure given below shows the energy barrier in this chemical reaction as a vertical hump.
Reactant molecules must gain a minimum amount of energy, called the activation energy Ea, in order to transform to product molecules.
- The relatively high energy barrier indicates that only the most energetic ozone molecules can react to form oxygen molecules.
- Chlorine atoms lower the energy barrier, which means more reactant molecules have sufficient energy to form product. Chlorine allows the reaction to proceed in two steps, and the two smaller energy barriers correspond to these steps (Note that the conversion is to write the catalyst above the reaction arrow).
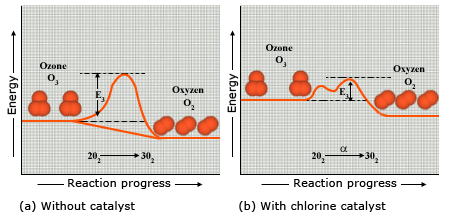
The conversion of ozone, O3, to oxygen, O2, is normally sluggish because the reaction has a relatively high – energy barrier.
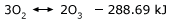
Ozone formation is endothermic. Energy is required to start this reaction.However, when chlorine atoms act as a catalyst, the energy barrier is lowered, and the reaction is able to proceed faster. Atomic chlorine lowers the energy barrier of this reaction by providing an alternate pathway involving intermediate reactions, each having lower activation energy than the uncatalyzed reaction. This alternate pathway involves two steps. Initially, the chlorine reacts with the ozone to form chlorine monoxide and oxygen
The chlorine monoxide then reacts with another ozone molecule to re–form the chlorine atom as well as produce two additional oxygen molecules
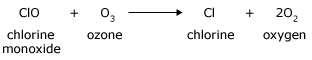
Although chlorine is used up in the first reaction, it is regenerated in the second reaction. As a result, there is no net consumption of chlorine. At the same time, however, a total of two ozone molecules are rapidly converted to three oxygen molecules. Chlorine is, therefore, a catalyst for the conversion of ozone to oxygen because the chlorine increases the speed of reaction but is not consumed by the reaction. Chlorine atoms in the stratosphere catalyze the destruction of the earth's ozone layer. Chlorine atoms are generated in the stratosphere as a by – product of human – made chlorofluorocarbons (CFCs), once widely produced as the cooling fluid of refrigerators and air – conditioners. Destruction of the ozone layer is a serious concern because of the role that this layer plays in protecting us from the Sun's harmful ultraviolet rays. One chlorine atom in the ozone layer, it is estimated, can catalyze the transformation of 100,000 ozone molecules to oxygen molecules in one or two years, before the chlorine atom is removed by natural processes. We are able to harness the power of catalysts for numerous beneficial purposes. The exhaust that comes from an automobile engine, for example, contains a wide assortment of pollutants, such as nitrogen monoxide, carbon monoxide, and un–combusted fuel vapors (hydrocarbons). To reduce the amount of these pollutants entering the atmosphere, most automobiles are equipped with a catalytic converter, shown in the Figure below. Metal catalysts in a converter speed up reactions that convert exhaust pollutants to less toxic substances. Nitrogen monoxide is transformed to nitrogen and oxygen, carbon monoxide is transformed to carbon dioxide, and unburned fuel is converted to carbon dioxide and water vapor. Because catalysts are not consumed by the reactions they facilitate, a single catalytic converter may continue to operate effectively for the lifetime of the car.
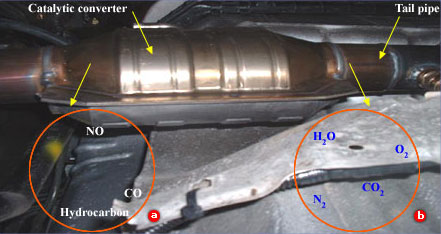
A catalystic converter reduces the pollution caused by automobile exhaust by converting such harmful combustion products as NO, CO, and hydrocorbons to harmless N2, O2, and CO2. The catalyst is typically platinum'Pt, palladium ' pd, or rhodium'Rd.
- Before the exaust reaches the catalytic converter it contains such pollutants as NO, CO, and hydrocarbons.
- After the exhaust has passed through the catalytic converter it contains water vapor, N2, O2, and CO2.

The exahust from automobiles today is much cleaner than before the advent of the catalytic converter, but there are many more cars on the road. In 1960 there were 70 million registered motor vehicles in the United states.In 2000 there were more than 200 million. The chemical industry depends on catalysts because they lower manufacturing costs by lowering required temperatures and providing greater product yields without being consumed.