Heating of electric currents
Ex 1:
An electric kettle is connected to 220 V mains supply. It took 5 minutes for the water in kettle to reach boiling point. How long would it take to boil the same amount of water, if the supply had been 120 V?
Sol:
The total electrical energy supplied to boil water in the kettle is W = VIt
Since nothing is clearly mentioned about the source of electrical power,
we assume that the power source is the
same, that means the electrical energy obtained
from the power source is
the same,
i.e the
current is same.
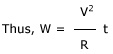

R
is the resistance of the coil in the electric
kettle.
We have to calculate t
The time t taken to boil the same amount of water for 120 V
The time t taken to boil the same amount of water for 120 V
supply is equal to
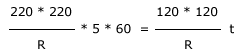
Solving this equation we get,
t = 1008.33s = 16.8
minutes.
Ex 2:
A potential difference of 220 V is applied across a resistance of 1 kiloohm. Calculate the heat energy produced in 10 seconds.
Sol:
The heat energy
produced can be calculated from
H = I2Rt
joules.
Here I
can be calculated from Ohm‘s law V =
IR, V = 220V and R = 1 kiloohm
I=V/R=220/1000 = 0.22 A
From this H
= 0.22 × 0.22 × 1000 × 10 = 484 J
Ex 3:
Calculate the temperature rise in 10 kg water when a 2 kW
electric heater is switched on for 15 minutes. (Specific heat of water C
= 4200J/kg/ oC).
Sol:
Power consumed
by the electric heater P = 2 kW
Time t = 15 minutes = 15/60 hours = 0.25 hours
Heat energy H = P × t = 2 × 0.25 kW h = 0.5 kW h
Convert kWh to
joules using the following equation:
1kW h = 3.6 × 106
joules
0.5 kW h = 1.8 × 106 joules
This is the energy used by the water heater to raise the temperature of water.
Heat used to raise the Temperature of water = Specific Heat of water (C) × Mass of water × Rise in Temperature ΔT
1.8
× 106
joules = 10 kg × C × ΔT
= 10 kg × 4200 J/kg/ oC × ΔT
⇒ ΔT = 42.85 oC
Thus the 10 kg
of water will rise by 42.85oC when a 2kW water
heats it for 15 minutes.
Assuming the initial temperature of water is room temperature
which is approximately
25oC,
we can see the final temperature of the 19 kg water will
be = 25o C + 42.85oC =
67.85oC