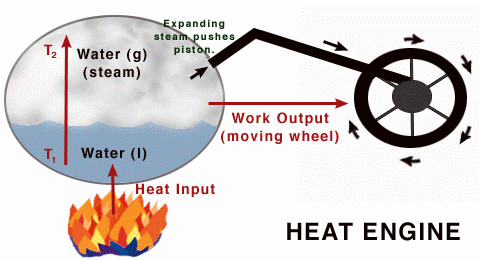
 Working of a Real Heat engine
As the steam (from water) pushes the piston, the wheel rotates, converting the heat energy given to the
system to work output.
Working of a Real Heat engine
As the steam (from water) pushes the piston, the wheel rotates, converting the heat energy given to the
system to work output.
A real engine must always reject some energy into the second heat reservoir in order to satisfy the second law of thermodynamics, so less energy is available to do external work, and the efficiency of the engine is reduced.
It is clear that real engines, which are always irreversible to some extent, are less efficient than reversible engines. Furthermore, all reversible engines which operate between the two temperatures T1 and T2 must have the same efficiency, irrespective of the way in which they operate.
A heat engine is a device that changes heat energy into mechanical work. It works on the first law of thermodynamics. The basic idea behind a heat engine, whether a steam engine, jet engine or internal combustion engine, is that mechanical work can be obtained only when heat flows from a high temperature to a low temperature. In every heat engine only some of the heat can be transformed into work.
The maximum efficiency of the real heat engine is about 57%. The real heat engine is not isolated as the matter enters and leaves the engine. Ideal cycles assume a closed system with the same working substance (air for instance), while the real engine exhausts air (as its oxygen content much decreased after burning) & fills with “another”. In a real engine, there must of course be a slight drop in temperature for the heat to flow.