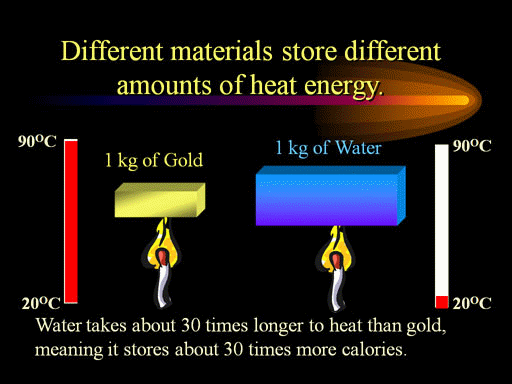
 The specific heat of gold is over 30 times smaller than that of water
One kilogram of gold will go from 20°C to 90°C, while water will only go from 20°C to 22°C when they both are heated equally.
The specific heat of gold is over 30 times smaller than that of water
One kilogram of gold will go from 20°C to 90°C, while water will only go from 20°C to 22°C when they both are heated equally.
The specific heat of any substance is the energy needed to raise the temperature of one gram of it by one degree Celsius.
The Molar Specific Heat in simple terms is the energy required to raise one mole of any substance by one degree Celsius.
Specific heats of gases are more complicated than of solids and liquids because, there may be large changes in pressure and volume for only a slight change in temperature. The specific heat of a gas depends very much on how the process of changing the temperature is carried out. We define Specific heats of Gas at constant pressure and volume.
Specific heat of a gas at constant volume [Cv] : It is defined as the amount of heat required to raise the temperature of 1 g of the gas through 1°C at constant volume. It is denoted by cv and is measured in cal g−1 K−1 (or) J kg−1 K−1.
Specific heat of a gas at constant pressure [Cp] : It is defined as the amount of heat required to raise the temperature of 1 g of the gas through 1°C at constant pressure. It is denoted by cp and is measured in cal g−1 k−1 (or) J kg−1 K−1 here we need to note that Cp > Cv.