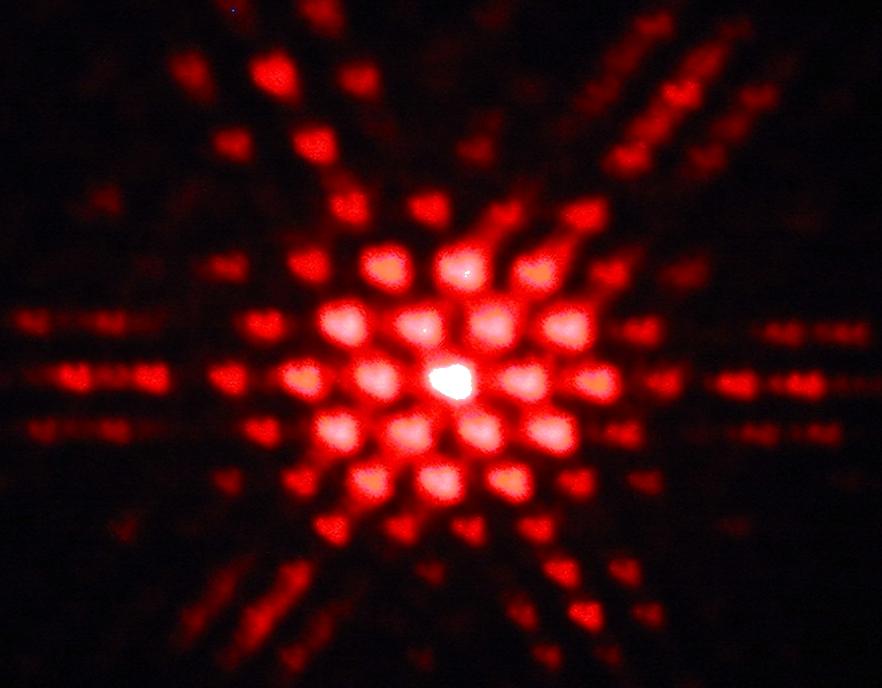
 The diffraction pattern from a hexagonal–shaped hole
This pattern proves the wave nature of electrons
The diffraction pattern from a hexagonal–shaped hole
This pattern proves the wave nature of electrons
Light is a form of energy which sometimes behaves as waves and sometimes as particles(photons).
Matter also can behave both like particles as well as waves. The concepts like Interference, Diffraction and polarization tell us that light is a wave. Experiments like photo–electric effect, Compton effect, Black body radiations, X–ray spectra shows light in its particle nature. The wave associated with a moving particle is called matter wave or de–Broglie wave and controls the particle in every respect. The intensity of a matter wave at a point represents the probability of the associated particle (e.g. electron) being there. Therefore, if the intensity of matter wave is large in a certain region, there is a greater probability of the particle being found there.
The de–Broglie wavelength of a moving particle is independent of the charge and nature of the particle. The greater the momentum (mv) of the particle, the smaller is the wavelength of the wave associated with it and vice–verse. The matter waves travel faster as compared to electromagnetic waves.