 Alpha Decay from a radio active nucleii
Alpha Decay from a radio active nucleii
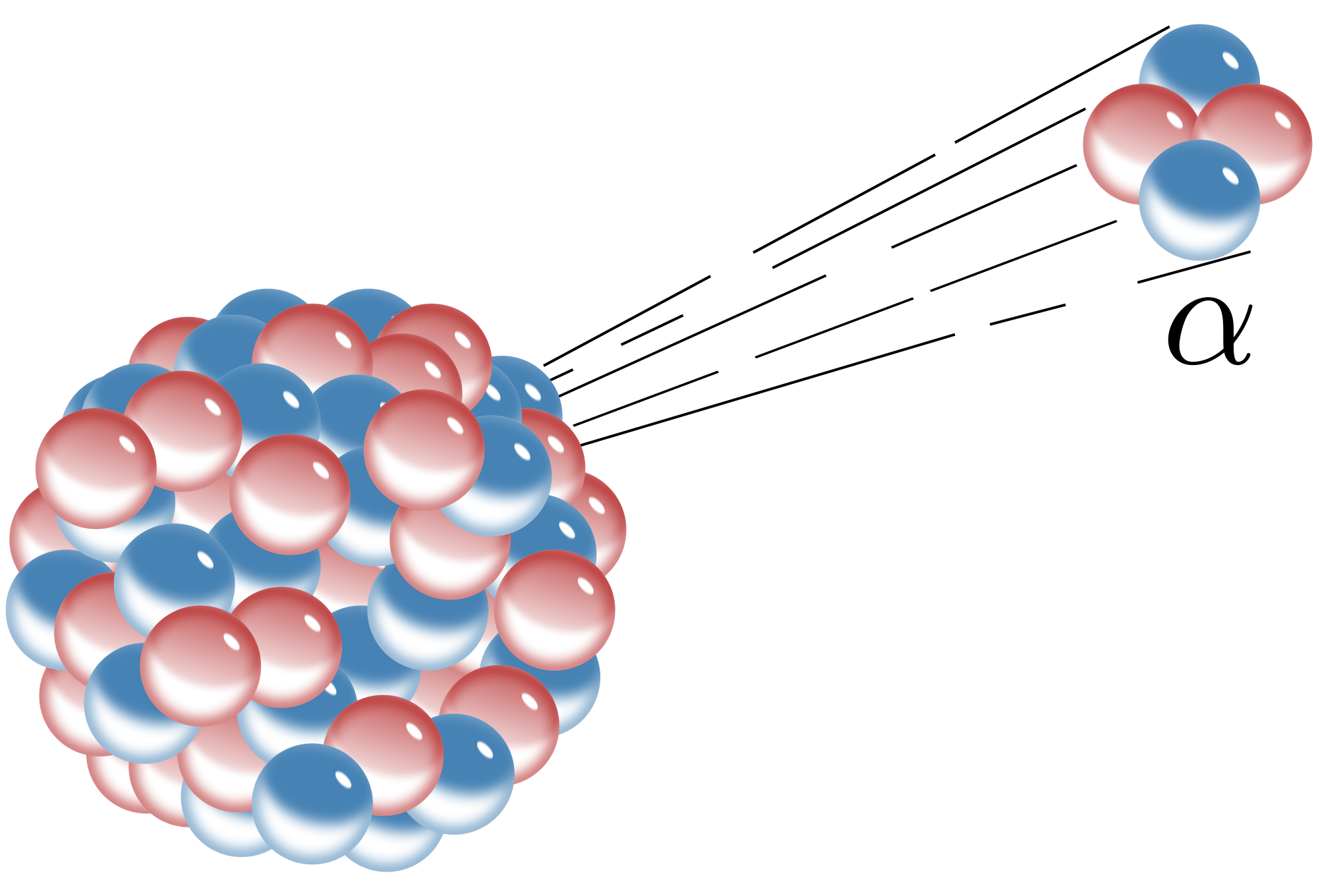
An alpha decay is one of three types of radioactive decay.
In this particular type, a particle made up of two neutrons and two protons is released. If you've noticed, the composition of this particle is similar to that of a Helium nucleus. This is the reason why some people simply describe alpha particles as Helium nuclei.
Of the three kinds of radioactive decay – alpha, beta, and gamma – it is the alpha decay that releases the most massive particle (the alpha particle). Because of its mass, an alpha particle cannot get far and may be stopped by a sheet of paper, a layer of skin, or even a few centimeters of air. Hence, it usually causes harm only when ingested. Alpha particles, just like betas and gammas, have ionizing characteristics. That is, they can expel an electron from an atom. This is the main reason why they can damage cells.
Alpha decay usually occurs when a radioactive nuclei is too heavy to be stable. In most cases, the nuclei can contain too many neutrons and protons. Thus, to attain stability, the nucleus gives off the two protons and two neutrons. In this process, since there is a change in the number of protons, i.e., the atomic number, the resulting element is different and lighter from the previous one.