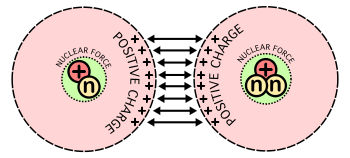
 Electrostatic repulsion between two nuclei
Electrostatic repulsion between two nuclei
The size of the nucleus is 10,000 times smaller than the size of the atom, but more than 99.9% of the whole mass of the atom is concentrated in the nucleus.
Therefore, a nucleus occupies a very small space in the atom consisting of protons and neutrons. Since neutron is a neutral particle, it has high penetrating power and very low ionizing power. Further, electric and magnetic fields have no effect on it. These two constituents of a nucleus (protons and neutrons) are called nucleons. The nucleus is believed to be spherical and hence its size is usually given in terms of radius. It has been found experimentally that the volume of a nucleus is directly proportional to its mass number A. In a usual practice the nuclear radius can be expressed in a smaller unit of length called Fermi.
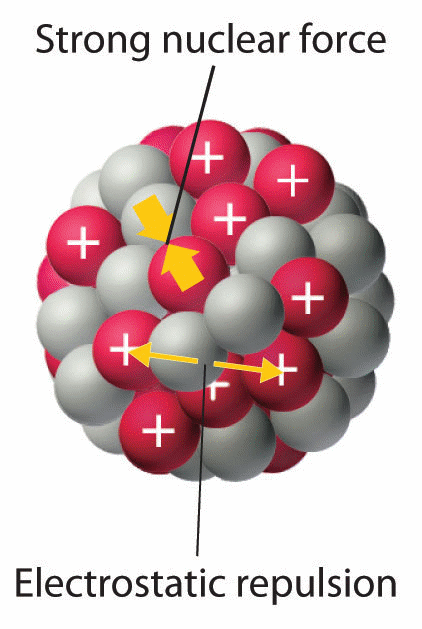
The strong forces of attraction which firmly hold the nucleons (i.e. protons and neutrons) inside the tiny nucleus are called nuclear forces. The nuclear forces exist between all the nucleons i.e. between a neutron and a proton, between two protons and between two neutrons.
Nuclear forces are short–range forces i.e. they act only over a short range of distances. The relative strengths of gravitational (FG), electrostatic (FE) and nuclear (FN) forces acting in the nucleus are: FG : FE : FN = 1 : 1036 : 1038. Nuclear forces are non–central forces. The force between two nucleons does not act along the line joining their centers. Nuclear forces are spin dependent. It has been found that the nuclear force between nucleons having parallel spins is greater than the nuclear force between nucleons having anti–parallel spins.