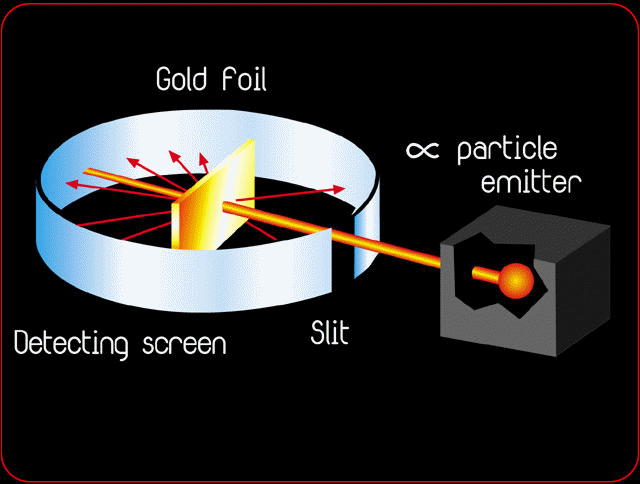
 Rutherford's Gold foil Experiment
Rutherford's Gold foil Experiment
Rutherford's model, based on the experimental results of Gold Foil, contained the features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom and with this central volume also containing the bulk of the atomic mass of the atom.
Those positively charged alpha particles deflected by large angles–some even backward, nearly in the direction from which they had come, which shows that there is a positive charge in center which is not distributed uniformly inside the atom.
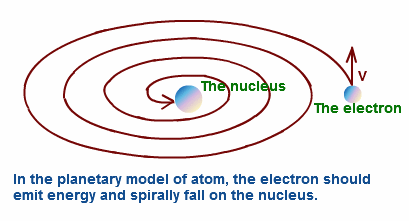 Limitations of Rutherford's Atomic Model
Limitations of Rutherford's Atomic Model
He also proposed that the electrons revolve around the nucleus in circular paths similar to the planets revolve around the Sun (solar system). The electrostatic force of attraction between the nucleus and electron provides centripetal force.
The radius of the nucleus was at least 1/10000 times smaller than that of the radius of the atom.
The quantity of charge, i.e., 1.602 × 10 −19 C is expressed as a unit charge, i.e., the charge of an electron is − 1 whereas that of a proton is +1.
Limitations of Rutherford's Atomic model:
1. This model could not explain how all the positive charge in the nucleus could stay without blowing itself apart.
2. As the electron revolves in a circular orbit, it is constantly subjected to centripetal acceleration and radiates energy
continuously ( as per Maxwell's EM theory) . As a result due to this continuous loss of energy, the electrons should
follow spiral path towards the nucleus and fall into it. Hence atoms must collapse, but they are stable.