 Production of electricity through steam generation is constructive power of nuclear energy.
Production of electricity through steam generation is constructive power of nuclear energy.
Nuclear chemistry is the study of reactions involving changes in the structure of the atomic nuclei. Modern nuclear chemistry has become very interdisciplinary in its applications, ranging from the study of the formation of the elements in the universe to the design of radioactive drugs for diagnostic medicine.
Radioactivity:
- If we look at all the nuclei that are present in nature, we find that a large number of heavy mass nuclei like uranium (U), thorium (Th), radium (Ra) spontaneously decay to small mass nuclei.
- The process of decay by which one species of nuclei transform into another is called radioactivity or radioactive decay.
- The decay occurs with certain rules or laws of radioactivity. The decaying or unstable nuclei are called radioactive elements. Radioactivity can be either spontaneous or induced.
- Spontaneous radioactive elements occur naturally like uranium, thorium, radium, etc..
- Induced radioactivity is achieved by nuclear reactions, either by fusing two small nuclei (fusion reaction) or fission or breaking up larger nuclei (fission reaction). For example, all transuranic elements are man made elements beyond uranium are radioactive.
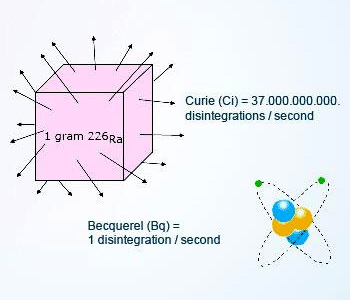 Units of radioactivity, Curie(Ci) and Becquerel(Bq)
Units of radioactivity, Curie(Ci) and Becquerel(Bq)
Units of radioactivity:
The common unit used for the measurement of radioactivity is curie (Ci). A curie is defined as the activity in one gram of
naturally occurring Radium–226 and equals to 37,000,000,000 disintegrations per second.
In the international system of units (SI), the Becquerel (Bq) is the unit of radioactivity. One Bq is 1 disintegration per second
(dps). One curie is equal to 37 billion Bq.
Conversion between curies and Becquerels:
1Ci = 3.7 × 1010 Bq = 37 GBq
1 mCi = 3.7 × 107 Bq = 37 Mbq
1 μCi = 3.7 × 104 Bq = 37 kBq
1 nCi = 37 Bq
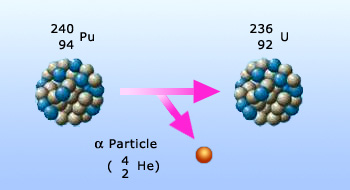 α – particle emission
α – particle emission
Radioactivity is categorized as:
- Alpha particles (α – particles): These are positively charged nucleus of helium 42He.
- Beta particles (β – particles): These are negatively charged electrons or positively charged positrons.
- Gamma rays (γ – rays): These have no charge and are electromagnetic radiations. Very rarely a radioactive atom may also emit neutrons.
Properties of α – particles:
- α – particles are nuclei of ‘He’.
- They produce intense ionization of the surrounding material through which they pass.
- They are not very penetrating, as their mass is high.
- Being charged particles, they are deflected by both electric and magnetic fields.
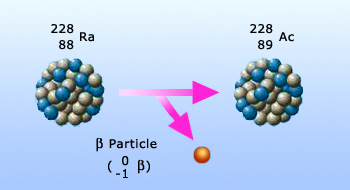 β – particle emission
β – particle emission
Properties of β – particles:
β− – particles are electrons. They are negatively charged. β+ – particles are
positrons. They are positively charged. Since the mass of the β – particles is small, their ionizing power while
passing through any matter is relatively small.
- The penetrating power of β – particles is larger than that observed for the α – particles. But the penetration is less than that seen for the β –rays.
- Since β – particles are charged particles, they can be deflected by electric and magnetic fields.
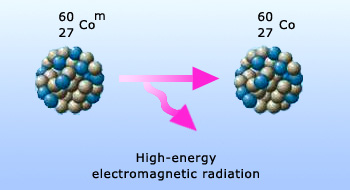 γ – particle emission
γ – particle emission
Properties of γ – particles:
γ–rays are photons or electromagnetic waves. Their wavelength is very small 10−10m. Emission of a
γ–ray does not change any of the A or Z or N numbers γ –rays have very high penetrating power, higher than
both the alpha rays and the beta rays.
- γ–rays have very low ionization power.
- Since they are uncharged, γ–rays cannot be deflected by either electric or magnetic fields.

Radioactive series:
The four different series of unstable heavy atomic nuclei (Given in adjacent figure) that decay through a sequence of alpha and
beta decays until a stable nucleus is achieved. These four chains of consecutive parent and daughter nuclei begin and end among
elements with atomic numbers higher than 81, which is the atomic number of thallium(Tl).Three of the sets, the Thorium series(4n),
Uranium series(4n+2) and Actinium series(4n+3), called natural or classical series.The fourth set, the neptunium series
(4n+1) is an artificial series headed by neptunium–237, its members are produced artificially by nuclear reactions and do
not occur naturally.
Thorium Series:
The Thorium (4n) series begins with 232Th and terminates with the formation of stable nuclide 208Pb. The
parent and each daughter nuclei in this series have a mass number that is divisible by 4 leaving the remainder of 0. This series
is also known as 4n series.
Example:
23290Th when 232 is divided by 4 remainder 0 is obtained making it a member of 4n series. In this, series of
transformations take place leading to the formation of 208Pb with the emission of 6α and 4β particles.
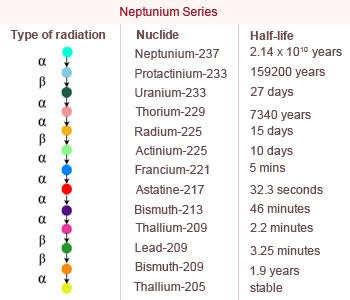
Neptunium Series
Members of the hypothetical Neptunium (4n + 1) series do not occur in nature because no long–lived parent is available.
The Neptunium (4n +1) series begins with 237Np and terminates with the formation of stable nuclide 207Tl.
Example:
22588Ra when 225 is divided by 4 remainder 1 is obtained making it a member of 4n + 1 series. In this,
series of transformations take place from 23793Np leading to the formation of 20581Tl
with the emission of 8α and 4β particles.
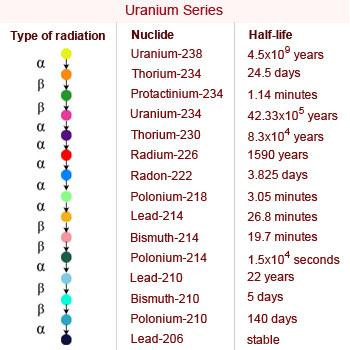
Uranium Series: The Uranium series (4n+2) starts with the isotope 238U and terminates with the formation of the stable nuclide 206Pb. The parent and each production in this series have a mass number that is divisible by 4 with remainder of 2. The uranium series is also known as the 4n + 2 series.
Example:
23892U when 238 is divided by 4 remainder 2 is obtained making it a member of 4n + 2 series. In this,
series of transformations take place leading to the formation of 206Pb with the emission of 8α and 6β
particles.
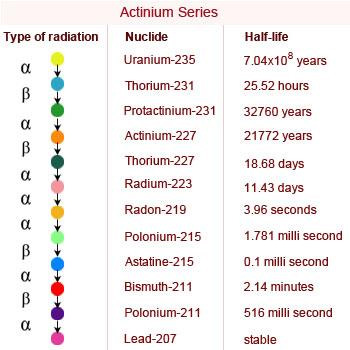
Actinium Series
The actinium (4n + 3) series begins with 235U and terminates with the formation of stable nuclide 207Pb.
The parent and each daughter nuclei in this series have a mass number that is divisible by 4 leaving the remainder of 3. This
series is also known as 4n + 3 series.
Example:
23592U when 235 is divided by 4 remainder 3 is obtained making it a member of 4n + 3 series. In this,
group series of transformations take place leading to the formation of 206Pb with the emission of 7α and 4β
particles.